Table 3.
Toward synthesis of 1,5-disubstituted triazoles.a
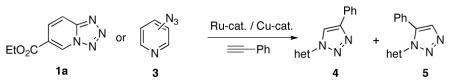 | ||||
|---|---|---|---|---|
| no. | substrate | catalyst | 4 | 5 |
| 1 | RuCpCl(PPh3)2 | - | - | |
| 2 | RuCp*Cl(PPh3)2 | - | - | |
| 3 | (CuOTf)2C6H6 | 81% | - | |
| 4 | RuCp*Cl(PPh3)2 | 23% | 36%b | |
| 5 | (CuOTf)2C6H6 | 91% | - | |
| 6 | RuCp*Cl(PPh3)2 | 3% | 56% | |
| 7 | (CuOTf)2C6H6 | 87% | - | |
Isolated yield. Reaction conditions: 5 mol % catalyst, 1,4-dioxane 0.25 M, 110°C (entries 1, 2, 4 and 6); 10 mol % catalyst, PhMe 0.25 M, 100°C (entries 3, 5 and 7).
Inseparable mixture of 4 and 5 (1:1.5).
