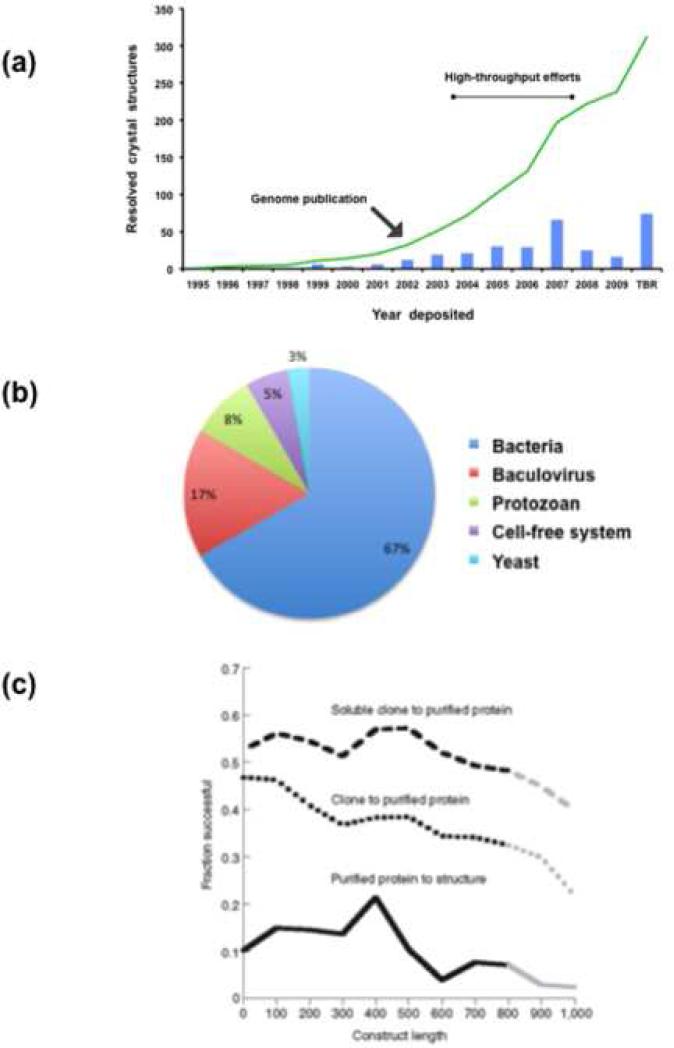
Figure 1.
(a) Resolved crystal structures of Plasmodium genes. PDB database (www.pdb.org, May 2009) was searched for Plasmodium protein resolved structures and plotted against the year deposited (bars: number of structures deposited in a given year; line: cumulative structure deposited numbers). After the sequence of the genome, there were two high-throughput efforts that resulted in a limited increase in the number of structures resolved. Overall, recombinant protein production remains a bottleneck for many scientific endeavors. TBR: to be released. (b) Heterologous protein expression systems for parasites. A PubMed search of recombinant proteins from parasites in the past seven years reveals that prokaryotic systems are still the workhorse, followed by insect cells/baculovirus. Interestingly, protozoan organisms are used more often than yeast. Cell-free systems are just starting to take off. (c) Solubility as a function of construct length; Ref. [32] is the original source. The overall success rate for soluble recombinant proteins remains very low, especially for protein targets with lengths > 800 amino acids [32]. Dotted line, fraction of cloned targets resulting in successful large-scale purifications. Dashed line, fraction of soluble clones at a 1 ml scale resulted in pure protein at large scale. Solid line, fraction of purified proteins resulting in successful crystal structure determinations.