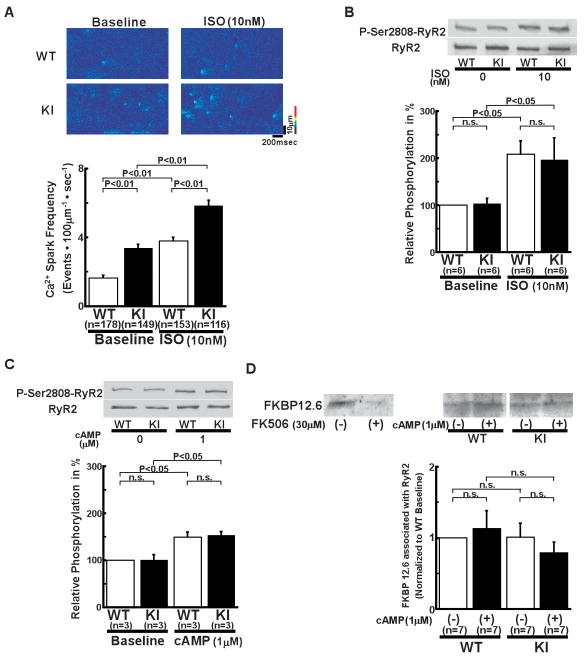
Figure 4.
Local Ca2+ release events in intact cardiomyocytes and Ser2808 phosphorylation in the RyR2. A, Spontaneous Ca2+ sparks. Top: Recordings of line-scan images of fluo-4 AM fluorescence with or without isoproterenol (10 nmol/L) in intact WT and R2474S/+ KI cardiomyocytes. Bottom: Summarized data of Ca2+ spark frequency. N: the number of cells from 6–8 hearts. B, Comparison of protein kinase A (PKA)-mediated phosphorylation level at Ser2808 of the RyR2 (P-Ser2808-RyR2) in intact KI and WT cardiomyocytes. In the presence of isoproterenol (10 nmol/L), KI and WT cardiomyocytes were incubated in the lysis buffer, and centrifuged. Then, the supernatant fraction containing crude homogenate was used for the phosphorylation assay. C, Comparison of P-Ser2808-RyR2 in KI and WT cardiomyocytes. In the presence of cAMP (1 μmol/L), okadaic acid (1 μmol/L), and CaMKII inhibitor KN-93 (1 μmol/L), KI and WT cardiomyocytes were incubated in the lysis buffer, and centrifuged. Then, the supernatant containing crude homogenate was used for phosphorylation assay. N: the number of cell lysate from 3–5 heats. D, Effect of cAMP on the RyR2-FKBP12.6 association in WT and KI cardiac homogenates. (Top) Representative Western blots of the RyR2 and FKBP12.6 after immunoprecipitation (cf. online supplemental methods). (Bottom) Summarized data of the ratio of FKBP12.6 to the RyR2, which was normalized to control condition. Although FK506 dissociated FKBP12.6 from the WT RyR2, shown as positive control, cAMP (1 μmol/L) did not dissociate the RyR2-bound FKBP12.6 in WT and KI cardiac homogenates. N: the number of SR preparations from 7–14 hearts.