Figure 4.
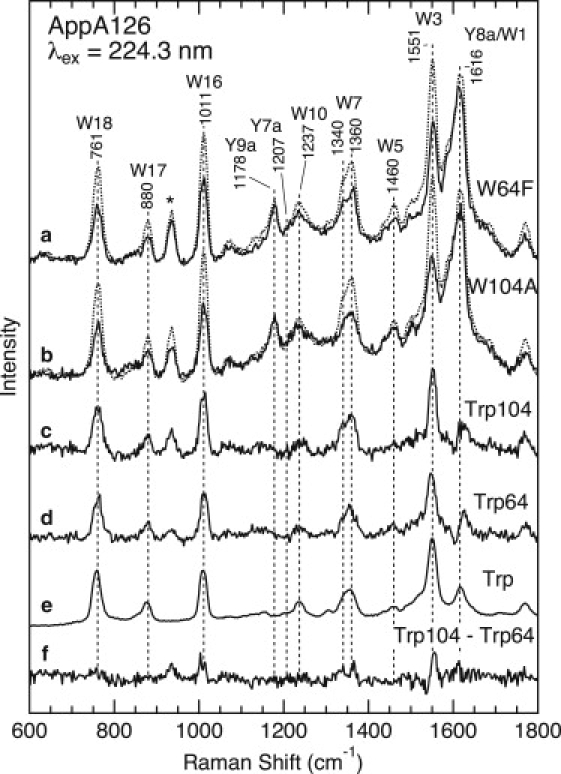
UVRR spectra of WT AppA126 and the W64F and W104A mutants in the dark state with 224.3 nm excitation. (a) WT AppA126 (dotted line) and the W64F mutant (solid line). (b) WT AppA126 (dotted line) and the W104A mutant (solid line). Estimated UVRR spectra for Trp-104 (c) and Trp-64,(d) as well as a spectrum of aqueous tryptophan (e), are also shown. The protein concentration was ∼50 μM and the buffer composition was 5 mM Tris-HCl, 1 mM NaCl, pH 8.0, 0.1 M NaClO4. The concentration of tryptophan was 1 mM, and the sample was dissolved in 10 mM Tris-HCl, pH 7.4. Trace f is the Trp-104 (c) minus Trp-64 (d) difference spectrum. The asterisk indicates a Raman band of ClO4−.
