Figure 2.
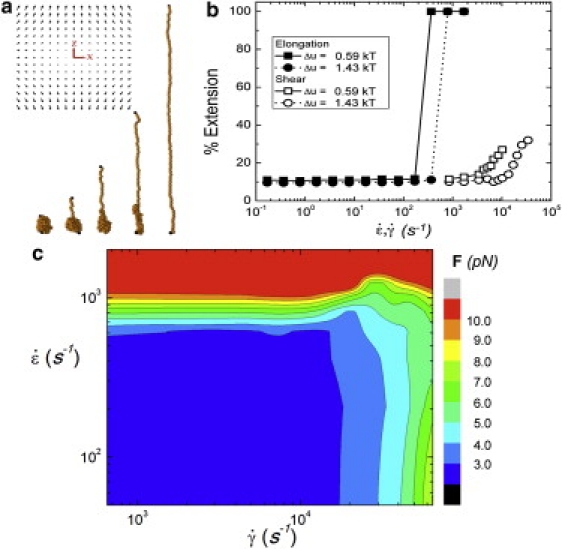
(a) The elongational flow (profile shown: z is the stretching axis as per Eq. 2 and Fig. 1, whereas x is an arbitrary transverse axis) imposed on a collapsed vWF molecule has the ability to stretch the protein. This opens up the quaternary structure to allow for bonding with other molecules. The snapshots shown represent the unfolding over time seen in simulations of vWF in elongational flow. (b) The average percent extension of vWF as a function of the elongation/shear rate for simulation using two different interaction energies Δu and a dimer size of ∼50 nm (see text for discussion). (c) The maximum tensile force along the chain F mapped as a function of elongation versus shear rates (u = 1.5 kT). We note that this maximum, for an extended chain, occurs at the center of the chain as predicted in Zhang et al. (3). This force quickly increases into the biologically relevant regime as elongational flow is applied, whereas application of shear flow demonstrates a much more broad transition.
