Figure 2.
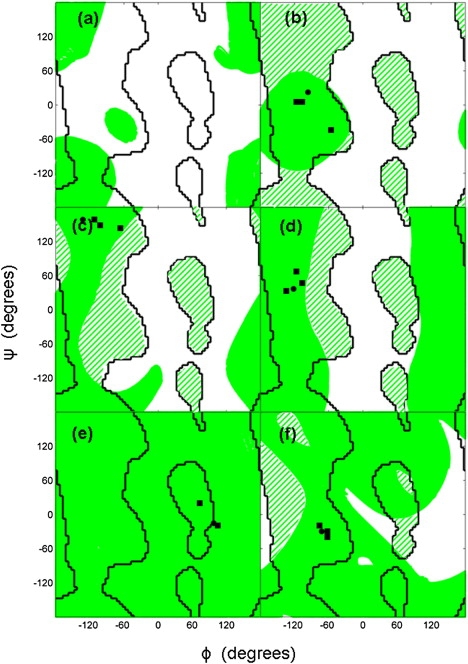
Starting from pentapeptide structures, the two-dimensional null space was explored as explained in the Supporting Material. The green areas in this Ramachandran plot show the regions visited by ϕ,ψ angles of residue 3 (for residues 2 and 4, see Fig. S2 and Fig. S3, respectively), the black lines are the boundaries between favorable and disfavorable regions, and the patterned regions show favorable regions that are inaccessible due to the constraint. Also shown are points indicating the ϕ,ψ angle of the residue in the starting structure (solid black circle) and from other structures in the same class (solid black squares), as given at the loop database website (http://www.bmm.icnet.uk/loop/index.html). (a) Starting from an extended conformation with ϕ,ψ angles (−123,136) at all residues, and a pentapeptide α-helix segment with ϕ,ψ angles (−57,−47). (b) Starting from loop α-α 1.1.5 (using the Oliva et al. classification code, PDB code 1ECA, segment 49–53A) (c) α-β 1.2.5 (PDB code 5P21, segment 137–141A) (d) β-α 3.1.1 (PDB code 2TMD, segment 395–399A). (e) β-β link 2.1.1 (PDB code 1EFT, segment 248–252A). (f) β-β hairpin 2.3.2 (PDB code 1HOE, segment 16–20A).
