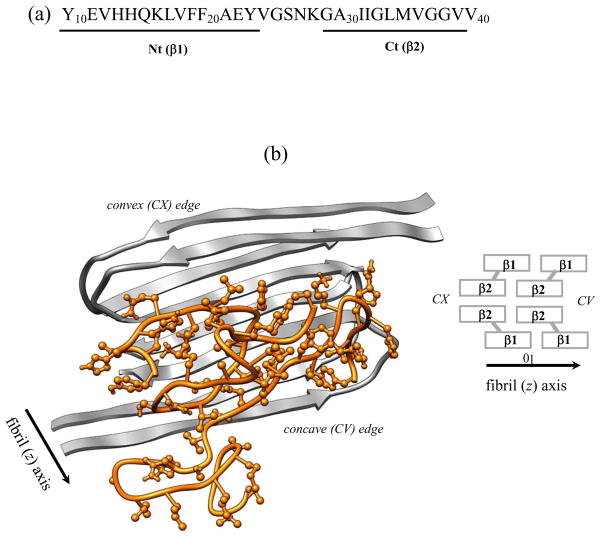
Fig. 1.
(a) The sequence of Aβ10–40 peptide and the allocation of the N-terminal Nt and C-terminal Ct regions. (b) Cartoon representation of the mutant Aβ10–40 hexamer. Fibril fragment includes four Aβ peptides in grey and consists of four parallel in-registry β-sheets formed by the β1 and β2 strands, which correspond to the Nt and Ct regions (panel (a)). Spacial allocation of β1 and β2 in the fibril gives rise to two distinct fibril edges - concave (CV) and convex (CX). Two incoming peptides in orange with side chains shown are bound to the CV fibril edge. The deletion of peptide-fibril backbone HBs in the mutant suppresses the formation of long β-strands in incoming peptides and disrupts their locking in fibril-like ordered conformations. The fibril structure is visualized using Chimera.58