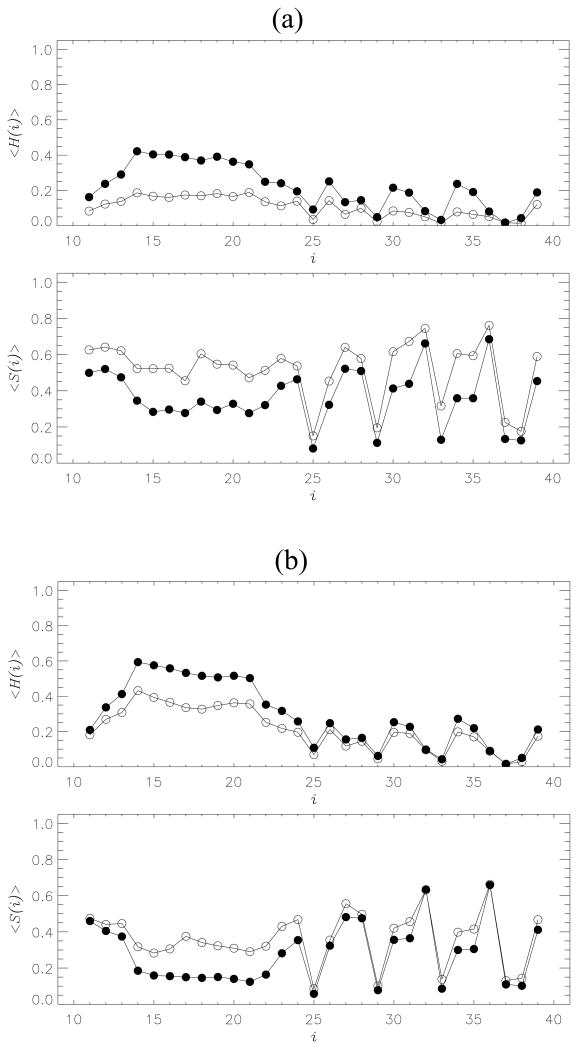
Fig. 5.
(a) Thermal distributions of the fractions of helix <H(i)> and β-strand <S(i)> structure formed by the residues i in Aβ10–40 peptides bound to the fibril: the MT (filled circles), the WT (open circles). (b) Distributions <H(i)> and <S(i)> computed for Aβ10–40 dimer: the MT (filled circles), the WT (open circles). The deletion of backbone HBs in the MT induces strand-to-helix structural conversion in the dimers and incoming peptides. The distributions <H(i)> and <S(i)> are obtained at 360K.