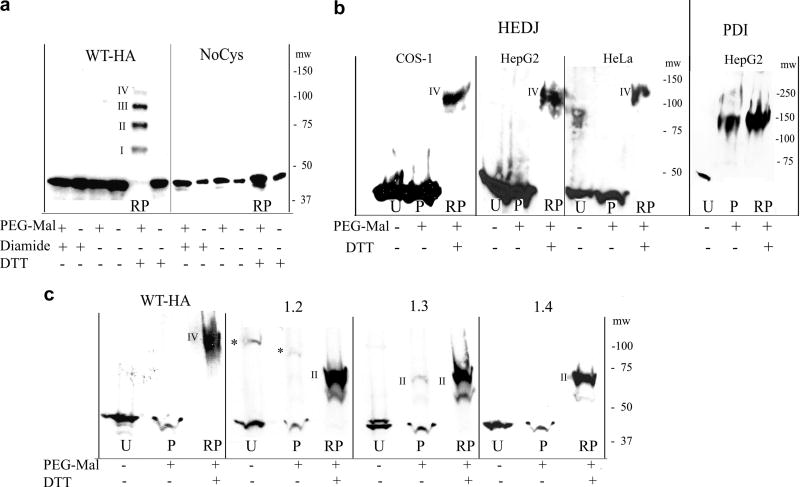
Fig. 2.
A. Microsomes, isolated from COS-1 cells that had been transfected with WT-HA or NoCys were reacted with 5 mM diamide, 100 mM DTT or untreated. They were TCA precipitated and treated with or without 5 mM PEG-Mal that had not been gel-filtered. Proteins were separated on a 7.5% non-reducing gel and immunoblotted with mouse anti-HA. In the lane labeled RP (DTT reduced + PEG-Mal), a ladder of bands approximately 15 kDa apart is seen, representing reduced, HA-tagged HEDJ conjugated to PEG-Mal on I, II, III or IV cysteines. The position of the molecular weight markers, mw, is shown on the right. B. HEDJ is predominantly oxidized in vivo. COS-1, HepG2 and HeLa cells were incubated with 20 mM DTT or untreated, TCA precipitated and the pellets processed as in A. except gel-filtered PEG-Mal was used and immunoblots were developed with an antibody to HEDJ or PDI. Lanes: U, untreated; P, untreated cells + PEG-Mal; RP, as in A (DTT reduced + PEG-Mal). The bands labeled IV in the RP lanes, correspond to band IV in Figure 2A based on their apparent molecular weights (see the molecular weight markers, mw on each panel). (For clarity, lane RP from the HepG2 cells, second panel, was taken from a shorter film exposure.) To validate the reaction conditions, an immunoblot of PDI from the processed HepG2 cells is shown in the right panel. C. WT-HA and several of the double mutants of HA-tagged HEDJ are predominantly oxidized in COS-1 cells. Cells were transfected with one of the constructs as shown, processed as in B., and the immunoblots developed with mouse anti-HA. Constructs conjugated to two PEG-Mal molecules are marked II. * denotes multimeric HA-tagged HEDJ