Fig. 4.
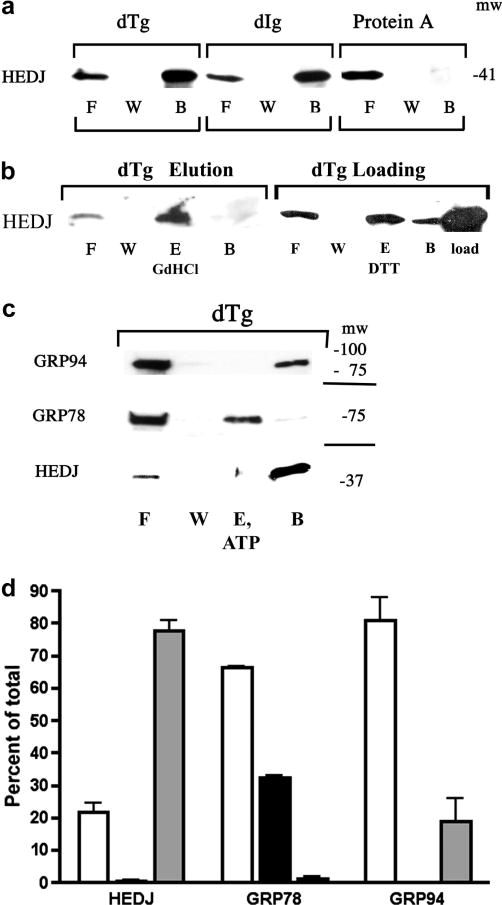
HEDJ can bind to immobilized dTg or dIg directly. A. Immobilized dTg or dIg or native protein A agarose beads were incubated with a microsomal lysate. The initial washes and flow through (F) and the final wash (W) were collected and concentrated. Material bound to the beads was released by boiling in SDS gel sample buffer (B). The proteins in all fractions were separated by using a 4–15% SDS-PAGE and immunoblotted with anti-HEDJ. B. In both immunoblots, binding assays to dTg were performed as in A. In the immunoblot labeled dTg Elution, an elution step (E, GdHCl) was included after washing the beads. The second blot, labeled dTg Loading, included a lane of unfractionated, unprocessed microsomes adjacent to the processed fractions in order to estimate total recovery. (See Figure 5 for explanation of the DTT elution shown in lane E.) C. GRP78 can be eluted from dTg using ATP while HEDJ and GRP94 cannot. The binding assay to dTg was performed as in B., using 2 mM ATP in the elution buffer. D. Quantification of C. For each assay, the total amount of recovered protein (density of the signals) was quantified and the percent of the total present in each fraction was determined. The value of each fraction is given as the mean +SEM. Open columns represent F, black columns represent E, and grey columns represent B. HEDJ (n=3); GRP78 (n=3); GRP94 (n=2). The percent of the total GRP78 that was recovered in the elution fraction was significantly different from the percent of HEDJ or GRP94 in the elution fraction. (p<0.0001