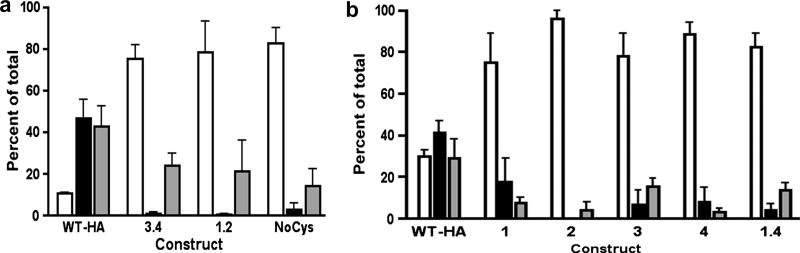
Fig. 6.
Cys mutants of HEDJ bind poorly to denatured protein. A. Immobilized dTg was incubated with lysed microsomes isolated from cells expressing wild type (WT-HA) or mutant (NoCys, 3.4, 1.2) HA-tagged HEDJ. The elution buffer contained 20 mM DTT. Proteins were separated by using a reducing 10% SDS-PAGE, immunoblotted using mouse anti-HA and quantified. Column legend: Open =F; black= E; gray = B. The differences between the amount of WT-HA and the mutants in (F) and (E) are statistically significant. (p<0.05, n=3) The proportion of WT-HA that remained bound to dTg (B) was significantly higher than that of the NoCys mutant (p<0.05, n=3). B. Quantification of the binding of single Cys mutants and the double mutant 1.4 to dTg. Assays were performed and fractions labeled as in A. There was a statistically significant difference in the proportion of recovered WT-HA that bound to the column as compared to each mutant. (P<0.05, one tailed P value) n=2.