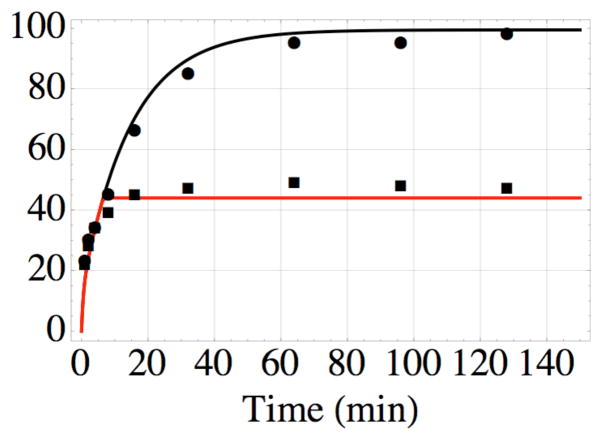
Figure 5.
Formation of the covalent bond between AABD(Y) and the G54C mutant of DOTA- binding Fab 2D12.5 in vitro at 37 °C, pH 7.5, as a function of time; nonlinear least-squares fit of data from reference 11 using equations 1–4. The top data (●) are for bond formation in the absence of competitor, while the bottom data (■) are the result of adding excess unlabeled competitor after 6 min. The values kon = 3.5× 106 M−1s−1 and koff = 7.0× 10−3 s−1 determined in this work lead to the nonlinear least-squares value kirr = 2.5 × 10−2 s−1 for formation of the covalent bond, and probability x = 0.2 of binding in a reactive conformation, all of which were used to construct the black curve. The red curve was drawn using the same values, with the added constraint of no association or reassociation after 6 min.