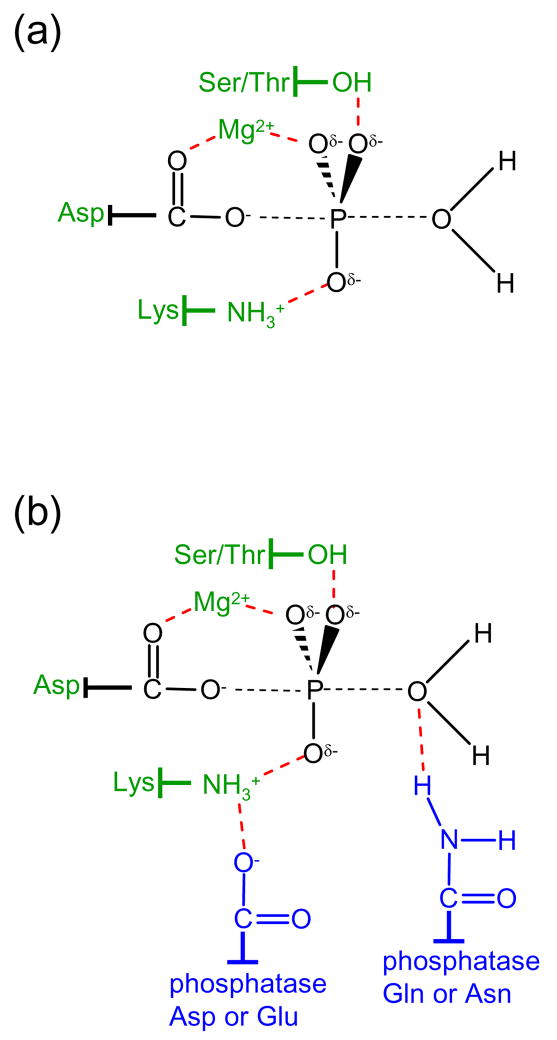
Figure 1.
Chemistry and catalysis of response regulator dephosphorylation. (a) The proposed bipyramidal transition state for hydrolysis of the aspartyl phosphate group is shown in black with partial bonds represented as dashed lines. Conserved groups proposed to catalyze response regulator autodephosphorylation are green and stabilizing interactions (deduced from structures of response regulators complexed with BeF3−) are represented by red dashed lines. (b) The same coloring scheme applies as in panel (a) but with the addition of conserved residues (blue) provided by phosphatases within the CheZ and CheC/CheX/FliY families. The conserved amide stimulates the reaction by direct interaction with the nucleophilic water molecule thus orienting it for the in-line attack. In CheX-mediated dephosphorylation, a threonine residue on CheY (Thr81 in B. burgdorferi CheY3) also forms a hydrogen bond with the attacking water molecule (not shown).