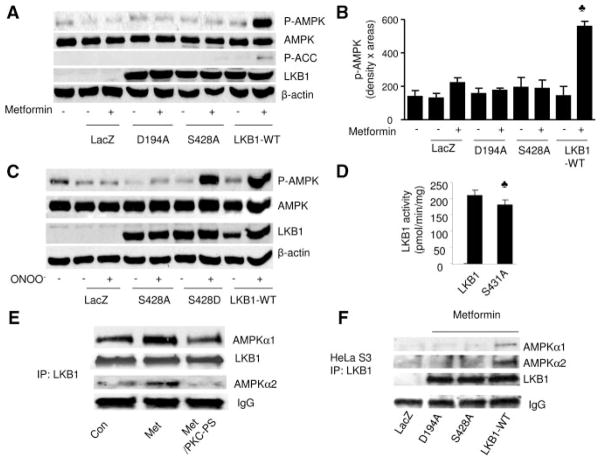
Figure 7.
Ser428 phosphorylation of LKB1 is required for metformin-enhanced AMPK activation and coimmunoprecipitation of LKB1 with AMPK. A and B, Metformin activates AMPK in HeLa-S3 overexpressing LKB1-WT but not LKB1-Ser428A mutants. After being transfected with LKB1-WT or kinase-dead LKB1 mutants or the LKB1-Ser428A mutant, HeLa-S3 cells were exposed to metformin (1 mmol/L, 1 hour). AMPK activation was monitored in Western blots with the use of specific antibodies. The blot is a representative of 5 blots from 5 independent experiments. C, Ser428 phosphorylation of LKB1 is required for ONOO−-enhanced AMPK activation. After being transfected with LKB1-WT or LKB1-Ser428A (Ser428 was mutated into alanine, loss of function) or the LKB1-Ser428D (Ser428 replaced by aspartic acid, phosphorylation mimicking), HeLa-S3 cells were exposed to ONOO− (100 μmol/L). AMPK activation was monitored by Western blotting with the use of specific antibodies. The blot is a representative of 5 blots from 5 independent experiments. D, LKB1 activity in LKB1 WT or LKB1-Ser431A mutants. E, Metformin increases the association of LKB1 and AMPK. LKB1 was immunoprecipitated from BAEC, and AMPK was detected in Western blots. Inhibition of PKC-ζ attenuated metformin-enhanced association of LKB1 with AMPK. The blot is a representative of 5 blots from 5 independent experiments. F, Mutation of Ser428 of LKB1 into alanine (S428A) abolishes metformin-enhanced coimmunoprecipitation of LKB1 with AMPK. Metformin increased the coimmunoprecipitation of LKB1 with LKB1 WT but not with LKB1 mutant. The blot is a representative of 3 blots from 3 individual experiments.