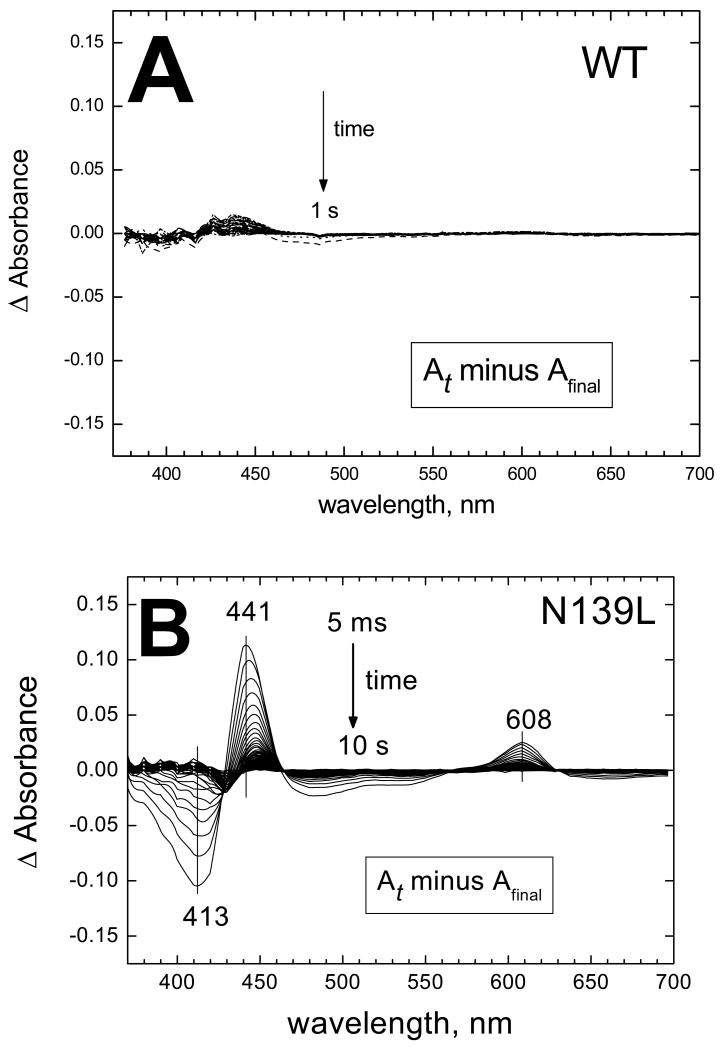
Figure 1. N139L replacement decelerates oxidation of dithionite-reduced enzyme by oxygen.
(A) Wild type enzyme. (B) N139L mutant. The WT and mutant enzyme were reduced anaerobically by 400 μM dithionite and mixed with oxygen in a stopped-flow diode array spectrophotometer. Spectra of the reaction mixture were taken each 4 ms for WT and 5 ms for N139L. The panels show difference absorption spectra versus the final spectrum of the oxidized enzyme that was taken as a baseline. Only 1 of each 10 spectra is shown in the figure. Final concentrations after mixing: COX, 5 μM, oxygen 500 μM. For other conditions, see Experimental procedures.