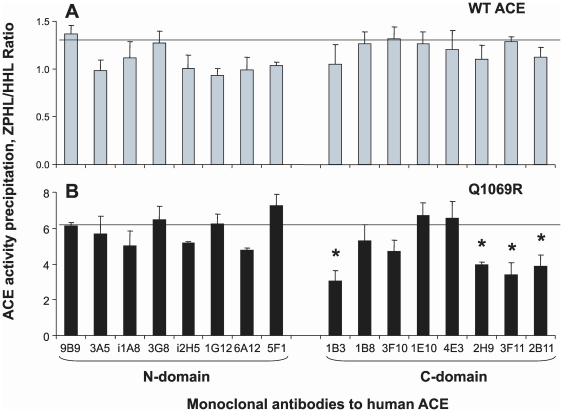
Figure 9. Catalytic properties of mutant ACE bound to ACE mAbs.
Membrane-bound WT (A) and mutant ACE (B) lysates were normalized to achieve 5 mU/ml ACE activity with Z-Phe-His-Leu as substrate and incubated in microtiter plates covered by 16 mAbs to human ACE as in Fig. 7. ACE activity precipitated by each mAb was quantified by fluorimetric assay with two substrates (Hip-His-Leu and Z-Phe-His-Leu) as in Fig. 5. Data are expressed as the ratio of ACE activity precipitated by each mAb determined with each substrate. Data are mean ± SD of 6–8 independent experiments in duplicate. * - p<0.05 vs. ZPHL/HHL ratio for corresponding values of WT and mutant ACE in solution (horizontal lines in A and B). The ratio for duplicate samples of WT ACE or mutant ACE was approximately 1.0 and the SD was less than 10%.