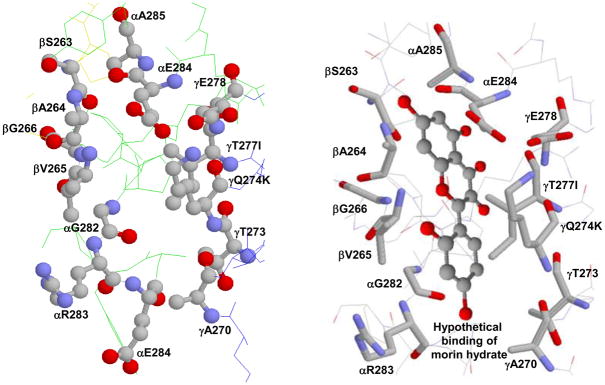
Figure 1.
X-ray crystallographic structure of polyphenol binding site of ATP synthase. (A) Empty and (B) hypothetical binding of morin hydrate at the polyphenol binding pocket. Residues from α, β, and γ subunits involved in interaction with polyphenols are identified. In bovine two variants, Q274K and T277I, occur in the γ subunit and are identified in the figure. PDB file 2jj1 [16] with RasMol [55] was used to generate this figure.