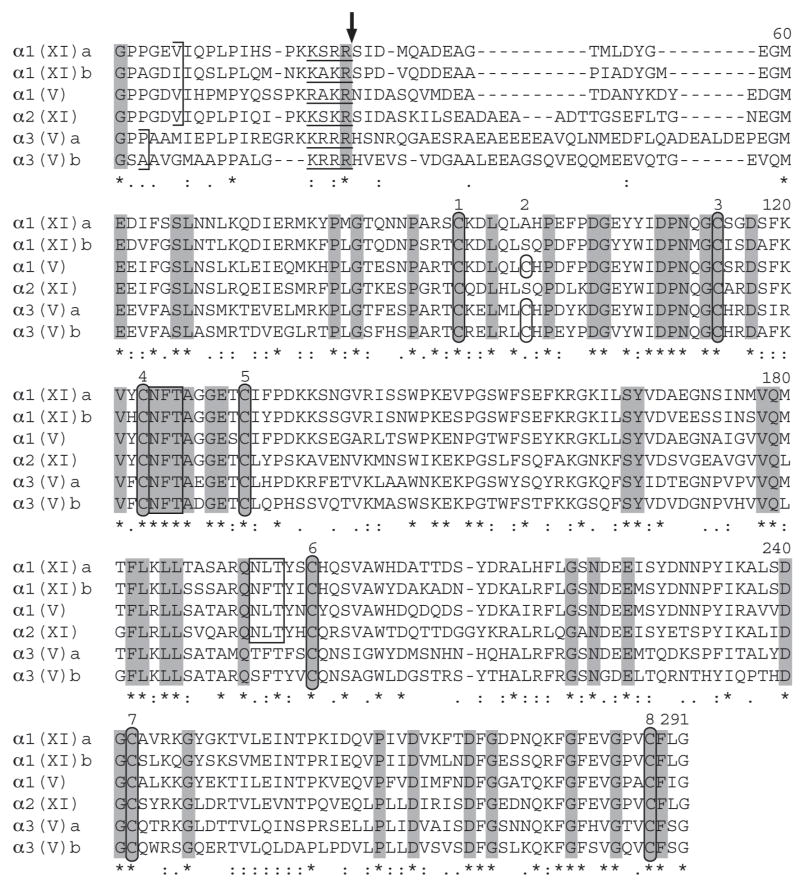
Fig. 6.
Alignment of C-propeptide sequences of zebrafish clade B procollagen chains. C-propeptide sequences were aligned using the EMBL-EBI ClustalW2 server. Dashes represent gaps introduced for optimal alignment. Ends of COL1 domains are marked by brackets. Consensus sites for cleavage by proprotein convertases are underlined, and a vertical arrow marks the predicted site for cleavage. Cysteines are circled and potential Asn-linked glycosylation sites are boxed. Asterisks and dots at the bottom of the alignment signify extent of similarity of aligned sequences, with asterisks denoting identity in all six zebrafish clade B chains. Residues identical in all six zebrafish and all reported mammalian clade B procollagen chains (Imamura et al., 2000) are shaded.