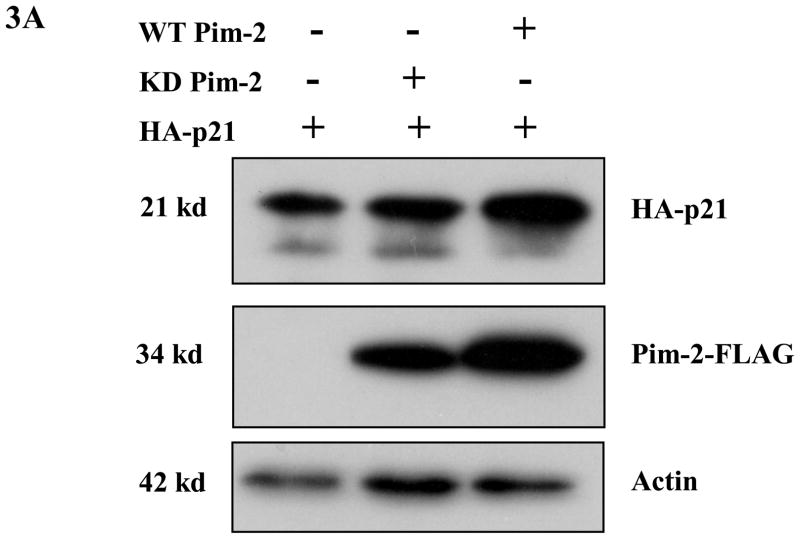
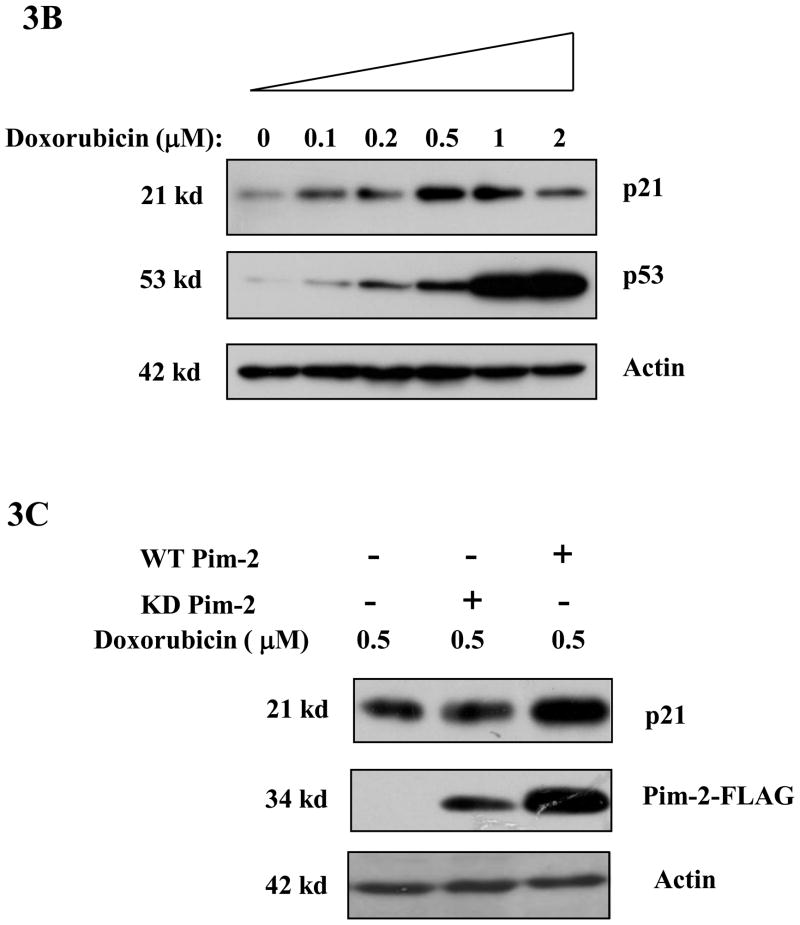
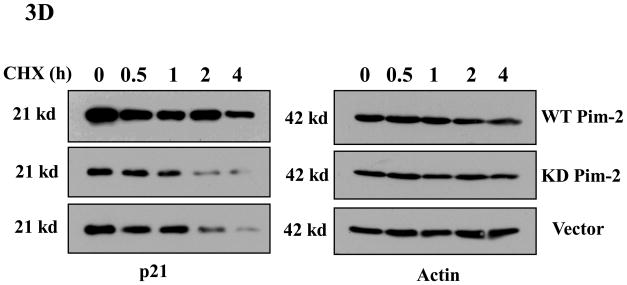
Figure 3. Pim-2 phosphorylation of p21 increases the stability of p21 proteins.
A) Pim-2 kinase activity increases the protein level of p21. HCT116 cells were co-transfected with HA-tagged p21 and either wild-type (WT) Pim-2-FLAG, Kinase dead (KD) Pim-2-FLAG or control vector. Forty hours after transfection, the protein levels of p21 were assayed by Western blotting with anti-HA antibody. Exogenous Pim-2 levels and control actin level were measured with anti-FLAG antibody and anti-actin antibody, respectively. B) Induction of p21 and p53 by increasing doses of doxorubicin in HCT116 cells. HCT116 cells were treated with the indicated amounts of doxorubicin for 24h. Induced p21 and p53 proteins were analyzed by Western blot with the designated antibodies. C) Exogenous Pim-2 kinase stabilizes p21 protein in doxorubicin-treated HCT116 cells. Either wild type (WT) Pim-2-FLAG, kinase dead (KD) Pim-2-FLAG or control vector was transfected into HCT116 cells. Doxorubicin was added at a final concentration of 0.5 μM to induce endogenous p21 after transfection. Cell lysates were then analyzed via Western blot for the expression of p21, Pim-2 and actin. D) Pim-2 kinase stabilizes p21 proteins. HCT116 cells were co-transfected with p21 and either WT Pim-2, KD Pim-2 or control vector. Forty hours post transfection, cells were treated with 25 μg/ml cycloheximide (CHX) and harvested at the indicated time points. The amount of degradation of p21 and actin proteins was analyzed by Western blot with anti-p21 and anti-actin antibodies.