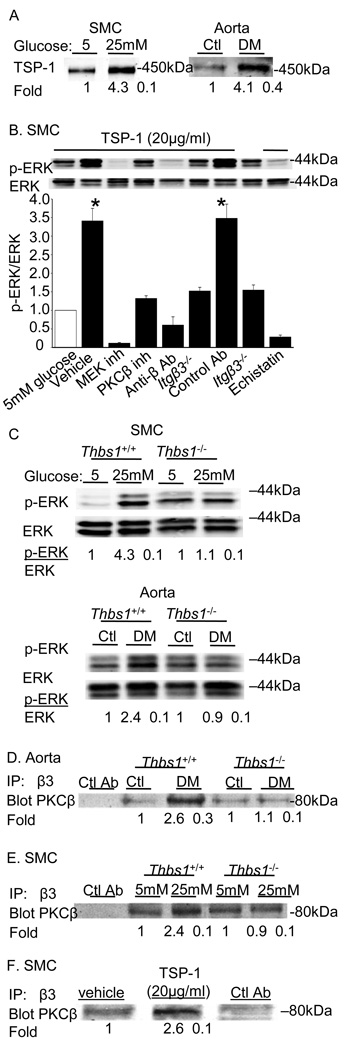
Figure 4. Role for the β3 integrin ligand thrombospondin (TSP-1) in the effects of hyperglycemia on SMCs.
Immunoblots from aortas isolated from control or diabetic mice or SMCs cultured under euglycemic (5 mM) or hyperglycemic (25 mM) conditions. The fold increase in TSP-1 levels relative to the values in euglycemic conditions are presented as the mean ± SE from three experiments (A). Exposure of SMCs cultured under euglycemic conditions to TSP-1 triggers ERK activation in a PKCβ and integrin β3-dependent manner. The results are presented as the level of phosphorylated/total protein normalized to results obtained in euglycemic conditions and are graphed as the mean ± SE from three independent experiments (B). Diabetic mice lacking TSP-1 (Thbs1−/−) do not upregulate ERK activation; similarly, Thbs1−/− SMCs do not upregulate ERK activation when cultured in 25 mM glucose (C). No increase in PKCβ is observed in integrin β3-immunoprecipitates from aortas of diabetic Thbs1−/− mice (D) or Thbs1−/−cells cultured in 25 mM glucose (E). The addition of TSP-1 to SMCs cultured in euglycemic conditions (5 mM) promotes the association of PKCβ with integrin β3 (F). Inhibitors used were as follows: MEK (U0126, 10µM), PKCβ (3-(1-(3-Imidazol-1-ylpropyl)-1H-indol-3-yl)-4-anilino-1H-pyrrole-2,5-dione, 5nM), integrin (echistatin, 10µM) and anti-β3 antibody (10µg/ml), control antibody (10µg/ml). Results are representative of those obtained in 3 independent experiments. *P<0.001 as compared to corresponding control.