Table 14.
Resolution of 3-substituted cyclic allylic alcohols.
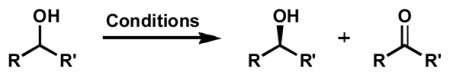 | ||||||
|---|---|---|---|---|---|---|
| entry | alcohol, major enantiomer | conditions[a] | time | conv.[b] | alcohol ee[c] | s |
| 1 | 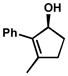 |
A | 4 h | 53% | 99% | 83 |
| 2 | B | 1 h | 53% | 98% | 63 | |
| 3 | D | 6 h | 55% | 94% | 26 | |
| 4 | 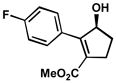 |
A | 4 h | 56% | 99% | 50 |
| 5 | B | 1 h | 56% | 99% | 51 | |
| 6 | C | 9 h | 51% | 95% | 83 | |
| 7 | 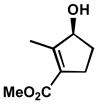 |
A | 8 h | 73% | 98% | 8.6 |
| 8 | B | 1 h | 61% | 85% | 9.0 | |
| 9 | C | 10 h | 78% | 99% | 7.5 | |
| 10 |
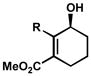 R = Me R = MeR = Bn |
B | 8 h | 65% | 99% | 15 |
| 11 | C | 27 h | 58% | 94% | 19 | |
| 12 | D | 43 h | 64% | 97% | 13 | |
| 13 | C | 72 h | 61% | 91% | 11 | |
See Table 10 footnotes.
