Table 3.
Added base in the kinetic resolution.
 | |||||
|---|---|---|---|---|---|
| entry | additive | time | conversion[b] | alcohol ee[c] | s |
| 1 | sparteine (0.3 equiv) | 13 h | 29% | 34% | 15 |
| 2 | none | 13 h | 2% | <2% | - - |
| 3 | Et3N (0.4 equiv) | 13 h | 26% | 31% | 22 |
| 4 | Et3N (0.4 equiv) | 26 h | 29% | 33% | 13 |
| 5 | Et3N (2.0 equiv) | 13 h | 19% | 19% | 11 |
| 6 | Et3N (4.0 equiv) | 13 h | 14% | 11% | 6 |
| 7 | DABCO (0.4 equiv) | 13 h | 7% | 8% | 20 |
| 8 | Na2CO3 (1.0 equiv) | 13 h | 27% | 31% | 15 |
| 9 | K2CO3 (1.0 equiv) | 13 h | 56% | 84% | 13 |
| 10 | Cs2CO3 (1.0 equiv) | 13 h | 68% | 99% | 13 |
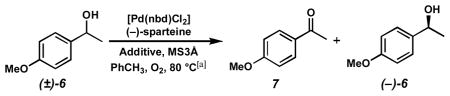
10 mol% [Pd(nbd)Cl2], 10 mol% (−)-sparteine, 1 atm O2, 500 mg MS3Å/mmol substrate, 0.1 M substrate in PhCH3.
Measured by 1H NMR.
Measured by chiral HPLC. See Supporting Information for details.
