Table 2.
Substrate scope
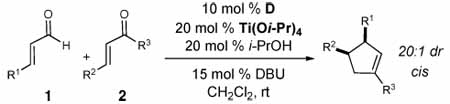 | |||||
|---|---|---|---|---|---|
| entry | R1 | R2 | R3 | % yielda | % ee cisb |
| 1 | Ph | Ph | Ph | 74 (3) | 99 |
| 2 | 2-naphthyl | Ph | Ph | 76 (4) | 99 |
| 3 | 4-Br-C6H4 | Ph | Ph | 67 (5) | 98 |
| 4 | 4-Cl-C6H4 | Ph | Ph | 65 (6) | 99 |
| 5 | 4-F-C6H4 | Ph | Ph | 62 (7) | 99 |
| 6 | 4-MeO-C6H4 | Ph | Ph | 80 (8) | 99 |
| 7 | 2-MeO-C6H4 | Ph | Ph | 54 (9) | 99 |
| 8 | 2-furyl | Ph | Ph | 73 (10) | 99 |
| 9c | n-propyl | Ph | Ph | 62 (11) | – |
| 10d | Ph | 4-Br-C6H4 | 4-Cl-C6H4 | 70 (12) | 99 |
| 11 | Ph | 4-Cl-C6H4 | 4-Cl-C6H4 | 67 (13) | 99 |
| 12 | Ph | 2-Cl-C6H4 | 4-Cl-C6H4 | 82 (14) | 99 |
| 13 | Ph | 4-F-C6H4 | 4-Cl-C6H4 | 70 (15) | 99 |
| 14d | Ph | 3-NO2-C6H4 | 4-Cl-C6H4 | 65 (16) | 99 |
| 15d | Ph | 4-Tol | 4-Cl-C6H4 | 60 (17) | 98 |
| 16d | Ph | 4-MeO-C6H4 | 4-Cl-C6H4 | 62 (18) | 99 |
| 17 | Ph | 2-furyl | 4-Cl-C6H4 | 67 (19) | 98 |
| 18 | Ph | 2-thienyl | Ph | 50 (20) | 98 |
| 19d | Ph | 4-pyridyl | Ph | 78 (21) | 99 |
| 20e | Ph | Ph | 4-Br-C6H4 | 81 (22) | 99 |
| 21d | Ph | Ph | 2-furyl | 75 (23) | 99 |
Isolated yield.
Enantiomeric excess determined by HPLC. Dr determined by 1H NMR (500 MHz) of the unpurified reaction.
15 mol % azolium salt A was used.
50 mol % Ti(Oi-Pr)4.
30 mol % Ti(Oi-Pr)4.
