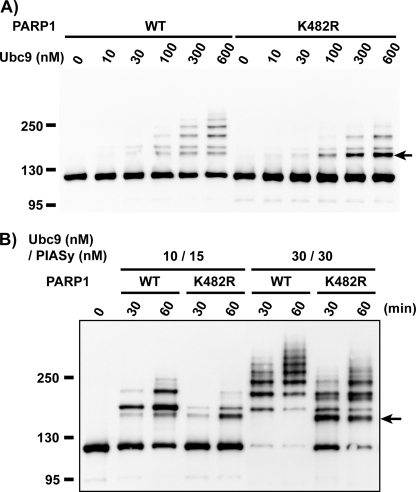
FIGURE 3.
The substitution of lysine 482 to arginine causes an alteration in the efficiency and specificity of the SUMOylation of PARP1 in in vitro SUMOylation assay. We have introduced a mutation at lysine 482 to arginine (PARP1 K482R) to confirm the mapping result. Purified PARP1 proteins were subjected to in vitro SUMOylation assay as in Fig. 3. A, shown is the Ubc9 dose-dependent reaction without PIASy. The SUMOylation reaction was performed with the indicated concentration of Ubc9 without PIASy. The reaction mixtures were incubated for 60 min. B, shown is the PIASy-dependent reaction. The reaction mixtures containing the indicated concentration of Ubc9 and PIASy were incubated for the indicated times. The arrow indicates the aberrant molecular mass shifted form that is more abundant in K482R mutant than the wild type.