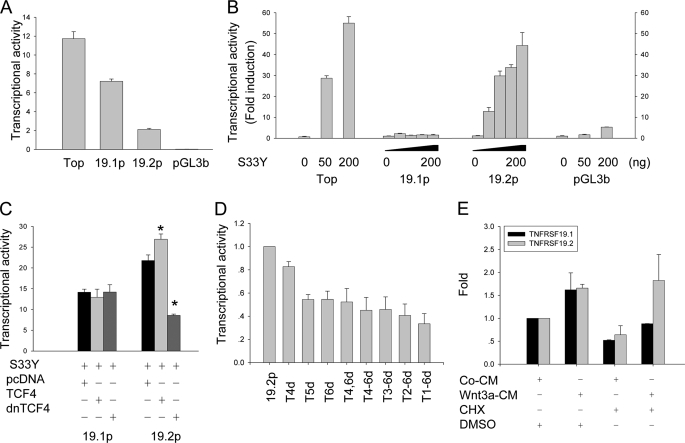
FIGURE 3.
TNFRSF19.2 is a direct target of canonical Wnt signaling. The promoter of TNFRSF19 transcript 1 (19.1p) and transcript 2 (19.2p) were cloned into the promoterless firefly luciferase reporter vector pGL3-basic (pGL3b). 293T cells were transfected with 50 ng of promoter firefly luciferase vector and 5 ng of pRL-TK Renilla luciferase vector as internal control by FuGENE 6. Topflash (Top) and pGL3b firefly vectors were used as positive and negative controls, respectively. A, basal transcriptional activity of TNFRSF19 promoters. B, dose-dependent activation of 19.2p by ectopic expression of a stable β-catenin S33Y (S33Y). 293T cells were co-transfected with 0, 25, 50, or 100 ng of S33Y for 19.1p and 19.2p analysis, and 0, 50, or 200 ng for controls. The data are presented as fold-induction compared with cells transfected without S33Y. C, dominant-negative TCF4 (dnTCF4) abolishes the activation of 19.2p by S33Y. 293T cells were co-transfected with 100 ng of S33Y and 200 ng of pcDNA, wild type TCF4, or dnTCF4 together with reporter vectors. D, proximal TBE are essential for Wnt activation. 19.2p harboring the single or multiple deletions of TBE were constructed and co-transfected with 50 ng of S33Y. The transcription activity of 19.2p was set to 1. The x axis indicates deletion of TBE in 19.2p. E, Wnt3a activates TNFRSF19.2 expression in the absence of protein synthesis. T253 cells were treated with 50% control condition medium (CM) or Wnt3a condition medium (Wnt3a-CM) with either dimethyl sulfoxide (DMSO) or 1 μg/ml of the protein synthesis inhibitor CHX for 8 h. Real-time RT-PCR was performed to detect TNFRSF19 expression, which was normalized against GAPDH. The expression level of TNFRSF19 in T253 cells treated with control CM and dimethyl sulfoxide was set to 1. All results are mean ± S.D. of three replicates. *, p < 0.01.