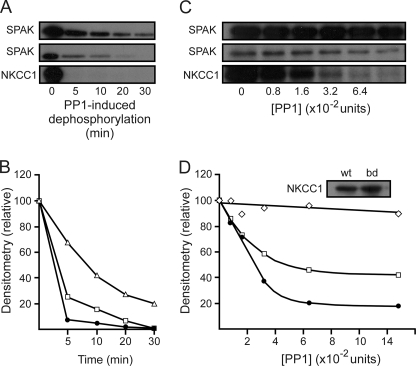
FIGURE 5.
Scaffolding of SPAK and PP1 increases the rate of kinase dephosphorylation. A, autoradiograph of a 45-min kinase reaction involving wild-type SPAK and wild-type NKCC1, followed by the addition of 2.5 units of recombinant PP1 for 5–30 min. One unit of PP1 hydrolyzes 1 nmol of substrate in 1 min. Upper panel, SPAK signal from reaction containing wild-type SPAK only; middle panel, SPAK signal from reaction containing both wild-type SPAK and wild-type NKCC1; bottom panel, NKCC1 signal from reaction containing both wild-type SPAK and wild-type NKCC1. B, densitometry analysis of the upper panel A (open triangles), middle panel A (open squares), and bottom panel A (filled circles). C, autoradiograph of a 45-min kinase reaction involving wild-type SPAK, wild-type NKCC1, and SPAK binding-deficient NKCC1 (F79A and F135A), followed by the addition of specified units of recombinant PP1 for 10 min. Upper panel, SPAK signal from the reaction containing both wild-type SPAK and SPAK binding-deficient NKCC1; middle panel, SPAK signal from reaction containing both wild-type SPAK and wild-type NKCC1; bottom panel, NKCC1 signal from reaction containing both wild-type SPAK and wild-type NKCC1. D, densitometry analysis of upper panel C (open diamonds), middle panel C (open squares), and bottom panel C (filled circles). Western blot analysis demonstrates that both wild-type NKCC1 (wt) and SPAK binding-deficient NKCC1 (bd) were present in sufficient amounts in their respective experiments (D, inset). Assays were repeated twice with similar results.