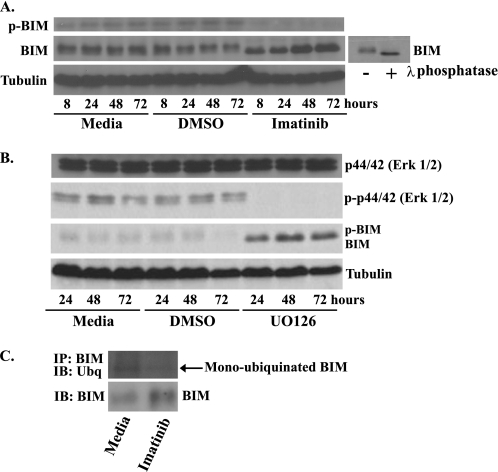
FIGURE 3.
BIM is dephosphorylated and deubiquitinated after treatment with imatinib in GIST 882 cells. Western blot analysis of whole-cell lysates prepared from GIST 882 cells following treatment with medium, DMSO, 1 μm imatinib (A) or 10 μm MAPK inhibitor UO126 (B). Blots were probed with antibodies specific to BIM, phospho-BIM, phospho-Erk1/2, Erk1/2, and tubulin (loading control). The small blot shows a GIST 882 whole-cell lysate that was treated with λ-phosphatase to generate a standard for the faster migrating, dephosphorylated form of BIM. C, GIST 882 cells were treated for 24 h with 1 μm imatinib, and then BIM was immunoprecipitated (IP) from cell lysates. The degree of BIM ubiquitination was determined by Western blot (IB) followed by probing with an anti-ubiquitin (Ubq) antibody. The amount of BIM in the original cell lysates was also determined by Western blotting.