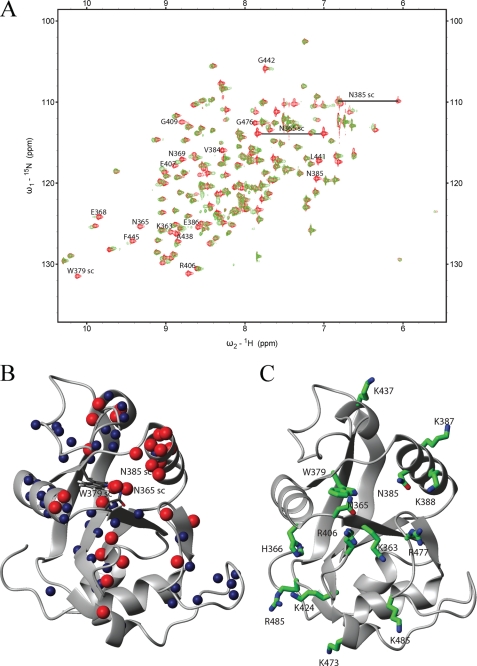
FIGURE 3.
NMR analysis of the YTH domain. A, overlay of the 15N-HSQC spectra of the free domain (red) and in a 2:1 RNA-protein complex (green). A change in position or disappearance of a peak indicates a change in the chemical environment of the respective atoms due to direct binding of the corresponding residue or structural rearrangements upon binding. The identity of selected residues is indicated (sc: side chain). B, backbone or side-chain amides of residues, which either disappeared (red spheres) or showed a large chemical shift changes (blue spheres), were mapped on the NMR structure of the YTH domain solved by the Riken Structural Genomics and Proteomics Initiative (PDB code: 2YUD). These include besides the backbone amides also side-chain atoms from two asparagines and one tryptophan. C, residues proposed to be involved in RNA binding mapped on the structure of the YTH domain.