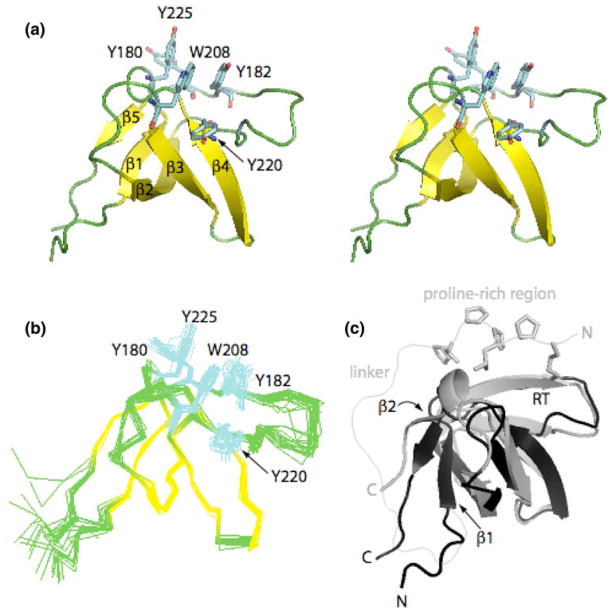
Fig. 2.
Itk SH3 domain structure. (a) Stereo view of the Itk SH3 average minimized structure. The five β strands that form the β barrel are colored in yellow while the loops are green. Aromatic residues located in the binding pocket: Y180, Y225, W208, Y182, and Y220, are colored in cyan. (b) Ensemble generated from the superposition of the 20 lowest energy structures on the backbone atoms (Cα, C′, N) for residues spanning 173–229. Color scheme and side chains shown are as in (a). (c) Overlay of the Itk SH3 and PrSH3 average minimized structures colored in black and gray, respectively. Structural differences between Itk SH3 and PrSH3 are evident in the β1, β2 strands, and RT loop. The side chains of the proline-rich region (KPLPPTP) are shown for the PrSH3 structure. The different N- and C-termini for the two structures are labeled and the linker within PrSH3 is indicated