Abstract
The molecular mechanisms of K+ homeostasis are only poorly understood for protozoan parasites. Trypanosoma brucei subsp. parasites, the causative agents of human sleeping sickness and nagana, are strictly extracellular and need to actively concentrate K+ from their hosts’ body fluids. The T. brucei genome contains two putative K+ channel genes, yet the trypanosomes are insensitive to K+ antagonists and K+ channel-blocking agents, and they do not spontaneously depolarize in response to high extracellular K+ concentrations. However, the trypanosomes are extremely sensitive to K+ ionophores such as valinomycin. Surprisingly, T. brucei possesses a member of the Trk/HKT superfamily of monovalent cation permeases which so far had only been known from bacteria, archaea, fungi, and plants. The protein was named TbHKT1 and functions as a Na+-independent K+ transporter when expressed in Escherichia coli, Saccharomyces cerevisiae, or Xenopus laevis oocytes. In trypanosomes, TbHKT1 is expressed in both the mammalian bloodstream stage and the Tsetse fly midgut stage; however, RNA interference (RNAi)-mediated silencing of TbHKT1 expression did not produce a growth phenotype in either stage. The presence of HKT genes in trypanosomatids adds a further piece to the enigmatic phylogeny of the Trk/HKT superfamily of K+ transporters. Parsimonial analysis suggests that the transporters were present in the first eukaryotes but subsequently lost in several of the major eukaryotic lineages, in at least four independent events.
Potassium (K+) is the most abundant cation in the cytosol of any cell and hence an essential macronutrient for life on earth. Concentrative K+ uptake across the plasma membrane is energized directly by ATPases and indirectly by the negative membrane potential or by coupling, via symport or antiport, to other transport processes such as H+ flux. The ancestry of K+ transporters renders them ideal subjects for phylogenetic comparisons. Indeed, the different kinds of known K+ transporters—pumps, channels, permeases, symporters, and antiporters—are all found in bacteria (43). Eukaryotes do not appear to have invented further mechanisms of K+ transport; on the contrary, some families of K+ transporters were lost over the course of eukaryote evolution, particularly among the metazoa (53).
The Trk/HKT superfamily (TC transporter classification 2.A.38 [43]) consists of bacterial TrkH and KtrB, plant HKT, and fungal Trk transporters (15). These proteins share a topology with 8 transmembrane (TM) domains and, sandwiched between odd- and even-numbered TM domains, 4 shorter hydrophobic helices that resemble the P-loops of K+ channels (14, 27, 55). In the K+ channel, these pore-forming loops end in the filter residues glycine-tyrosine-glycine, which coordinate K+ by means of their backbones’ carbonyl oxygens (13). The P-loop-like helices of Trk/HKT transporters end in a single conserved glycine (48), and these glycines have been shown to determine K+ selectivity over Na+ of the transporters (34, 49). Thus, a Trk/HKT monomer with 8 TM domains and 4 P-loops is thought to have a similar pore architecture to a K+ channel tetramer with two TM domains and one P-loop per subunit. The Trk/HKT transporters are important for cellular K+ acquisition in microorganisms, since trk null mutant yeast or bacteria exhibit growth phenotypes on media containing low K+ concentrations (20, 46). The roles of the Trk/HKT transporters in plants are more diverse, including Na+ distribution (10, 33, 47), osmoregulation (32), and salt tolerance (39). So far, no HKT/Trk transporter has been described from the metazoa or protista.
Trypanosoma brucei subsp. parasites comprise the causative agents of human and livestock trypanosomosis: sleeping sickness and nagana, respectively. The distribution of the parasites is restricted by that of their vector, the blood-sucking tsetse fly (Glossina spp.), to the so-called tsetse belt comprising 36 countries between the Sahara desert and the Kalahari (3, 8). African trypanosomes proliferate extracellularly in the blood, evading the mammalian immune response by antigenic variation. Untreated sleeping sickness is fatal. There is an urgent need for new and better drugs since the current ones, the arsenical melarsoprol in particular, suffer from severe side effects (31). In the mammalian bloodstream, the parasites encounter a rich and steady supply of nutrients, readily imported by specific permeases or endocytosed via receptors (7). Research on trypanosomal nutrient uptake has so far concentrated on transporters of organic substrates: nucleobases, nucleosides, sugars, and amino acids (4, 12, 26, 30, 35, 56). Little is known about how the parasites import inorganic nutrients. The malaria parasite Plasmodium falciparum possesses two putative K+ channel subunits with 6 TM domains and one P-loop (19, 52). Disruption of an orthologous gene in Plasmodium berghei strongly impaired the development of the malaria parasites in the mosquito (18). However, these putative channels have not yet been proven to be permeable to K+. The T. brucei genome (6) is annotated to contain two putative K+ channels; in addition, a putative ATPase has been identified resembling fungal Na+/K+ efflux ATPases (5, 45). None of these has been addressed experimentally. Here we present the identification and characterization of TbHKT1 (Tb10.70.2940), a Trk/HKT-type K+ transporter from Trypanosoma brucei and representative of a new clade of Trk/HKT genes from kinetoplastid parasites.
MATERIALS AND METHODS
In silico methods.
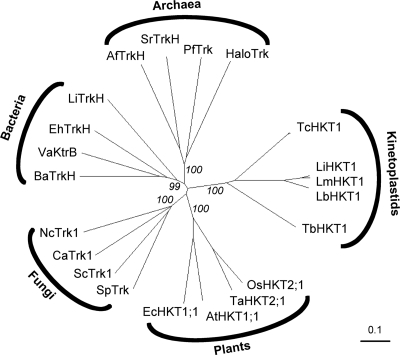
A Trk/HKT-specific profile (hkt.hmm; available on request) was made with HMMer (17) from a ClustalW multiple alignment (50) of a homology-reduced set of representative Trk/HKT proteins and validated against the proteomes of Arabidopsis thaliana and Saccharomyces cerevisiae. Parasite predicted proteomes were downloaded from ftp.sanger.ac.uk (T. b. brucei v4.0, Leishmania major, and Trypanosoma cruzi), www.plasmoDB.org (P. falciparum v5.5), ftp.ebi.ac.uk/pub/databases/integr8 (Theileria parva), ftp.tigr.org (Entamoeba histolytica and Trichomonas vaginalis), and www.giardiadb.org (Giardia duodenalis v1.1). The obtained hits were run through TMHMM (29) to predict membrane topology and searched against the GenBank nonredundant protein database with Blastp (2) to test for eventual relationships to other, non-Trk/HKT sequences. The phylogenetic tree (see Fig. 3) was drawn from a ClustalW alignment with Dendroscope (25). Bootstrapping was performed for 1,000 rounds, and results were expressed as percent positives.
Fig. 3.
Phylogenetic tree of the Trk/HKT superfamily with representative members. Bootstrap numbers of the main branches are shown in percent positives of 1,000 rounds. Protein abbreviations and GenBank accession numbers are as follows: T. cruzi TcHKT1 (71424637), L. braziliensis LbHKT1 (154344371), L. infantum LfHKT1 (146100295), L. major LmHKT1 (72546739), Pyrococcus furiosus PfTrk (18894043), Archaeoglobus fulgidus AfTrkK (11498445), Salinibacter ruber SrTrkH (83816848), Halobacterium HaloTrk (15791310), Arabidopsis thaliana AtHKT1;1 (7716474), Oryza sativa OsHKT2;1 (14588581), Triticum aestivum TaHKT2;1 (567062), Eucalyptus camaldulensis EcHKT1;1 (9719299), Leptospira interrogans TrkH (24193699), Vibrio alginolyticus KtrB (3395637), Enterococcus hirae TrkH (G53610), Bacillus anthracis TrkH (229604742), Candida albicans CaTrk1 (8099700), Neurospora crassa NcTrk1 (3724137), S. cerevisiae Trk1 (72015), and Schizosaccharomyces pombe Trk1 (1182049).
Cell lines and media.
Monomorphic bloodstream-form T. b. brucei 221 (MiTat 1.2; 221) parasites were cultivated at 37°C, 5% CO2 in HMI9 medium supplemented with 5% fetal calf serum (24). Procyclic T. b. brucei 427 (derived from MiTat 15a) parasites were grown at 27°C in SDM medium supplemented with 5% fetal calf serum (9). For RNA interference (RNAi) experiments, bloodstream-form T. b. brucei “New York single-marker” and procyclic T. b. brucei 29-13 were used (54). Transfectants were selected in 0.1 mg/ml puromycin and cultivated in the presence of 1 mg/ml tetracycline to induce transcription of the stem-loop RNAi construct (1). The rat L6 myoblast cell line was obtained from ATCC (CRL-1458) and cultivated at 37°C with 5% CO2 in RPMI 1640 medium supplemented with 2 mM l-glutamine, 5.95 g/liter HEPES, 2 g/liter NaHCO3, and 10% fetal calf serum. Yeast complementation experiments were performed with trk1- and trk2-deficient S. cerevisiae strain CY162 (20) on arginine/phosphate medium (41), consisting of 8 mM phosphoric acid, 10 mM l-arginine, 2 mM MgSO4, 0.2 mM CaCl2, 20 mg/liter histidine, 2% galactose, and 0.3% sucrose, supplemented with the standard vitamins and trace elements and 1.5% agarose (because agar contains too much potassium). KCl was added to the desired concentration. Escherichia coli complementation was performed with the trkD (kup1) trkG trkH kdpABC5 quadruple mutant LB2003 (46) grown in a medium containing 46 mM Na2HPO4, 23 mM NaH2PO4, 8 mM (NH4)2SO4, 0.4 mM MgSO4, 6 μM FeSO4, 1 mg/liter thiamine, 1% glucose, 0.25 mM isopropyl-β-d-thiogalactopyranoside (IPTG), and KCl to the desired concentration.
Drug sensitivity tests.
Trypanosomes (103 bloodstream forms per well, respectively, 104 procyclic forms per well) or L6 myoblasts (4 × 103 per well, seeded 24 h prior to the addition of test compounds) were cultivated in 100 μl medium on a 96-well plate containing serial dilutions of test compounds. After 70 h of incubation, 10 μl per well Alamar Blue solution was added (12.5 mg resazurin in 100 ml phosphate-buffered saline) and after another 2 h of incubation, fluorescence was measured with a Spectramax Gemini fluorimeter (Molecular Devices, Sunnyvale, CA) at 536 nm excitation and 588 nm emission. Fifty percent inhibitory concentrations (IC50s) were determined by nonlinear regression to a sigmoidal dose-response curve with Prism 5 (GraphPad Software, Inc.). Each assay was performed in triplicate and repeated at least 3 times. All chemicals were purchased from Sigma/Fluka.
FACS analysis.
Cells (8 × 106 procyclic forms [PCF] or 106 bloodstream forms [BSF]) were incubated with 100 mM K+-glutamate, 1.5 μM valinomycin, or both at 27°C (PCF) or 37°C (BSF) for 20 min. Then the cells were brought to a density of 1 × 106/ml, and 1 min before fluorescence-activated cell sorter (FACS) measurement, 400 nM the anionic DiBAC4(3) (bis-[1,3-dibutylbarbituric acid] trimethine; Molecular Probes) was added and flow cytometry was performed with a Becton Dickinson FACSCalibur (fluorescence window, 505 nm to 530 nm). The lower the membrane potential is (i.e., the more depolarized), the more the anionic fluorescent DiBAC enters the cell. For each measurement, 10,000 cells were analyzed. Data were evaluated with the CellQuest Pro software version 5.2.
Cloning and plasmid constructs.
TbHKT1 was amplified from genomic DNA of T. b. brucei 221 by PCR with the forward primer (GGGATCCACGCGATATGGCCAGTCAA; BamHI site underlined) and the reverse primer (GTCTAGAACTCACGGCAGCGAATTGA; XbaI site underlined). The product was cloned via the incorporated restriction sites into pYES2 for expression in S. cerevisiae, into pPAB404 for expression in E. coli, and into pOMU for in vitro transcription of cRNA. For RNAi-mediated gene silencing, a 280-bp fragment of TbHKT1 encompassing nucleotides 1489 to 1771 (codons 496 to 590) was amplified by PCR from genomic DNA of T. b. brucei with the forward primer GCAAGCTTGGATCCCGTACGCCATCAGACCATTCACCT (HindIII site underlined and BamHI site double underlined) and the reverse primer GCTCTAGACTCGAGTGCTGAAATCCACAGTATAGTGGCC (XbaI site underlined and XhoI site double underlined), and cloned into pGEM-Teasy (Promega). The insert was subcloned twice, in opposite directions separated by a 460-bp stuffer, into the vector pALC14 (1), a derivate of pLEW100 (54).
Gene expression analyses in trypanosomes.
Total RNA was extracted from trypanosomes by the “hot phenol” method. About 108 cells were lysed in a preheated (90°C) mixture of 50% phenol, 0.5% SDS, 45 mM NaCl, 4.5 mM Tris-HCl (pH 7.5), 2.3 mM EDTA, and centrifuged. After phenol-chloroform extraction, the RNA was ethanol precipitated, washed, and resuspended in diethyl pyrocarbonate (DEPC)-treated water. Total RNA (10 μg) was electrophoresed on a 1.2% agarose gel and blotted on a Nylon membrane (Roche Diagnostics). The RNA was probed with an α-[32P]dCTP-labeled TbHKT1 probe (Megaprime DNA labeling system; Amersham) and scanned on a PhosphorImager (Amersham Bioscience).
Electrophysiological recordings of Xenopus oocytes.
Capped cRNA of TbHKT1 and A. thaliana HKT1 (AtHKT1) was prepared in vitro with the mMESSAGE mMACHINE kit (Ambion, Austin, TX). Xenopus laevis oocytes were prepared as described previously (20, 27, 51). Oocytes were kept at 16°C in Barth's solution [88 mM NaCl, 1 mM KCl, 2.4 mM NaHCO3, 0.33 mM Ca(NO3)2, 0.41 mM CaCl2, 0.82 mM MgSO4, 10 mM HEPES-NaOH, pH 7.4]. Ionic currents were recorded 1 day after injection of cRNA (10 ng per oocyte) by two-electrode voltage clamping, in a bath solution containing 6 mM MgCl2, 1.8 mM CaCl2, the indicated concentrations of K+ and Na+ (as glutamate salts), 10 mM Mes-1,3-bis(tris[hydroxymethyl]methylamino)propane, pH 5.5, and osmolality adjusted to 240 mosmol/kg with d-mannitol. Voltage clamping and data acquisition were performed with voltage clamp amplifiers (Cornerstone model TEV-200 from Dagan Instruments, Minneapolis, MN; or AxoClamp 2B from Axon Instruments, Foster City CA).
RESULTS AND DISCUSSION
Phenotypic characterization of K+ homeostasis in T. brucei.
As a first step toward the characterization of the mechanisms underlying K+ homeostasis in Trypanosoma brucei, we measured their sensitivity to perturbation of [K+] in the medium and their susceptibility to K+ antagonists. The assays were performed in vitro, using the redox-sensitive dye Alamar blue as an indicator of cell viability (40). Both the mammalian-stage bloodstream forms and the tsetse fly midgut-stage procyclic forms of T. b. brucei were tested. The trypanosomes tolerated K+ concentrations of 100 mM and Na+ concentrations of 200 mM (Table 1), exceeding by far the physiological levels in human serum: 4.4 mM for K+ and 142 mM for Na+ (28). The values in Table 1 include the contributions of the medium, which were 5.8 mM K+ and about 144 mM Na+ for procyclic as well as bloodstream-form trypanosomes (the sodium concentration is difficult to estimate since the pH of the medium was adjusted with NaOH). The trypanosomes had millimolar IC50s for Rb+ and Cs+, albeit they were still an order of magnitude more sensitive to Rb+ than the rat myoblast cell line included as a reference (Table 1). Furthermore the trypanosomes were rather insensitive to the K+ channel blocker tetraethylammonium (TEA+), the bloodstream forms in particular, and they tolerated the channel-blocking charybdotoxin from scorpion venom at the highest applicable dose (Table 1). This apparent robustness of the mechanisms underlying K+ homeostasis in T. brucei contrasted with the trypanosomes’ extreme susceptibility to the K+ ionophores valinomycin and gramicidin (Table 1), the trypanosomes being over 1,000-fold more sensitive than the rat myoblasts. Valinomycin and gramicidin themselves may not be of therapeutic value due to their toxicity. Nevertheless, their trypanocidal potency indicates that K+ homeostasis in African trypanosomes, in spite of their insensitivity toward K+ analogs and channel blockers, harbors essential and sensitive drug targets.
Table 1.
Sensitivity of African trypanosomes to K+ analogs and antagonistsa
| Analog/antagonist | Sensitivity (IC50) for: |
||
|---|---|---|---|
|
T. brucei |
Rat L6 myoblasts | ||
| Procyclic forms | Bloodstream forms | ||
| Na+ | 240 mM | 264 mM | 291 mM |
| K+ | 150 mM | 154 mM | 124 mM |
| Rb+ | 8.5 mM | 4.1 mM | 82 mM |
| Cs+ | 7.9 mM | 14 mM | 15 mM |
| TEA+ | 12 mM | 70 mM | 31 mM |
| Charybdotoxin | >10 μM | >10 μM | >200 nM |
| Valinomycin | 1.4 nM | 170 pM | 200 nM |
| Gramicidin | 170 pM | 1.0 nM | 3.4 mM |
The cations were administered as chloride salts. IC50s were determined in vitro as described previously (40).
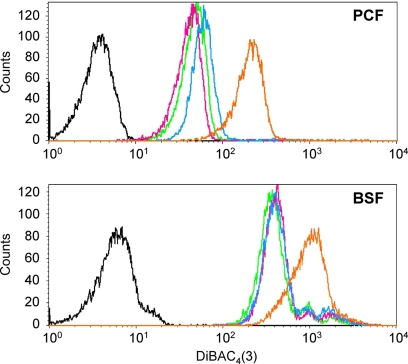
Cell viability being a rather crude indicator, we used the fluorescent bis-oxonol dye DiBAC4(3) to monitor by FACS analysis (fluorescence-aided cell sorting) the immediate effect of externally added K+ on the membrane potential in trypanosomes. DiBAC4(3) serves as a qualitative potentiometric probe since the influx of the dye is inversely proportional to the polarization of the target cell. Bloodstream-form T. brucei trypanosomes exhibited a higher fluorescence after addition of bis-oxonol (400 nM) than procyclic forms, indicating a less negative membrane potential (Ψp) in the bloodstream forms (green curves in Fig. 1). This is in agreement with previous studies reporting a membrane potential of −152 mV for procyclics (11) and −82 mV for bloodstream forms (37). The trypanosomes (bloodstream as well as procyclic forms) did not spontaneously depolarize in the presence of high (100 mM, added in the form of glutamate salt in order to prevent potential artifacts from Cl− currents) extracellular K+ concentrations (Fig. 1, pink curves). The effect of exogenous K+ on Ψp in T. brucei has been controversial (11, 37). Our results indicate that in T. brucei, bloodstream as well as procyclic forms, the plasma membrane is not immediately penetrable for K+. Addition of the K+ ionophore valinomycin (1 μM) alone did not affect Ψp (Fig. 1, blue curves), indicating that K+ is at electrochemical equilibrium at physiological external [K+]. Only upon addition of 100 mM K+ in combination with 1 μM valinomycin, did the cells depolarize within minutes (Fig. 1, orange curves). This was harmful to the cells, and they died after 50 min of incubation (not shown). Thus, although K+ is in equilibrium at resting membrane potential, there do not seem to be open K+ channels through which the cation could readily pass the T. brucei plasma membrane.
Fig. 1.
Trypanosomes do not spontaneously depolarize. T. b. brucei procyclic (top) and bloodstream (bottom) forms were incubated with the fluorescent dye DiBAC (colored curves; black curve, untreated control cells, no DiBAC) and the fluorescence was quantified by FACS analysis. Green, normal medium; pink, medium supplemented with 100 mM K+; blue, medium supplemented with 1 μM valinomycin; orange, medium supplemented with 100 mM K+ plus 1 μM valinomycin.
A new clade of Trk/HKT genes from trypanosomatids.
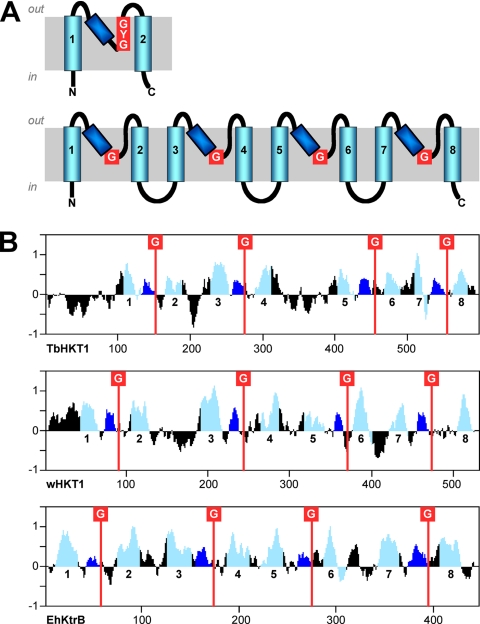
The T. brucei genome (6) encodes two putative K+ channels, Tb09.160.3380 and Tb927.1.4450. A further gene product, Tb927.10.16170, is annotated as a putative K+ channel in the TriTryp database (TriTrypDB.org), but while it has a predicted K+ channel tetramerization domain (Pfam profile PF02214), it is not predicted to possess transmembrane domains by TMHMM (29). In order to identify further K+ transporters from T. brucei, we screened the predicted proteome (version 4, 9192 proteins) with hidden Markov model-based profiles for various cation transporter families. The profiles were made with HMMer (16) from homology-reduced sets of representative transporters and validated against the predicted proteomes of Arabidopsis thaliana and S. cerevisiae. Based on these searches, African trypanosomes do not possess cyclic-nucleotide-gated cation channels, KUP/HAK K+ permeases (TC 2.A.72) or K+:H+ antiporters (TC 2.A.37), transporter families which are ubiquitous in plants (21). The only search that returned a significant hit (E-value of 1.5e−5) from T. b. brucei was with the Trk/HKT-specific profile; the identified protein, Tb10.70.2940, was named TbHKT1. The gene product Tb10.70.2940 has recently been renamed to Tb927.10.4300 (TriTrypDB.org). The similarity of TbHKT1 to known Trk/HKT proteins is rather low at the level of primary amino acid sequence; the founding member of the HKT family, TaHKT2;1 from wheat (44), was used as a reference in Table 2. The similarity becomes more evident when comparing hydropathy profiles (Fig. 2). TbHKT1 has an extended hydrophilic N terminus of 100 amino acids without similarity to any known sequence, followed by the typical topology of HKT/Trk proteins: four P-loop like domains, each between two predicted TM domains and each followed by a glycine residue (Fig. 2), the presumed K+ selectivity filter. TbHKT1 has orthologues in other trypanosomatids: two almost identical alleles in T. cruzi, one in Trypanosma vivax and in various Leishmania spp. (Table 2); only Trypanosoma congolense appears to lack a TbHKT1 orthologue. The respective genomic regions are syntenic in all trypanosomatids (TriTrypDB.org). These trypanosomatid HKTs form a new, distinct branch on the phylogenetic tree of the Trk/HKT superfamily, equally distant to the plant HKT and fungal Trk proteins (Fig. 3). There is no evidence for horizontal transfer of plant HKT genes to trypanosomes, as has been suggested for metabolic enzymes (22). In E. coli and other bacteria, the K+ translocator TrkH requires the NAD-binding subunit TrkA (36) and the ATP-binding subunit TrkE/SapD (23). However, HMMer searches with TrkA- and TrkE/SapD-specific profiles did not provide any evidence for such accessory proteins in T. brucei (not shown).
Table 2.
Predicted Trk/HKT proteins from trypanosomatids
| Species | Protein | TriTryp accession no. | Length (aa) | % similarity to TaHKT2;1a |
|---|---|---|---|---|
| T. b. brucei | TbHKT1 | Tb927.10.4300 | 599 | 29 |
| T. vivax | TvHKT1 | TvY486_1004280 | 610 | 26 |
| T. cruzi | TcHKT1 | Tc00.1047053511469.60 | 592 | 33 |
| Tc00.1047053506295.120 | ||||
| L. major | LmjHKT1 | LmjF35.0080 | 579 | 31 |
| L. brasiliensis | LbrHKT1 | LbrM34_V2.0120 | 580 | 31 |
| L. infantum | LinHKT1 | LinJ35_V3.0080 | 579 | 30 |
Percent similarity to TaHKT2;1 from wheat (Triticum aestivum) was measured by Needleman-Wunsch global alignments with Blosum62.
Fig. 2.
TbHKT1 carries the hallmarks of Trk/HKT transporters. (A) Schematic representation of the membrane topology of KcsA-type K+ channels (top) and Trk/HKT transporters (bottom). Transmembrane domains are depicted in light blue, P-loops in dark blue, and K+ selectivity filters in red. (B) Kyte-Doolittle hydrophobicity, colored according to the same scheme, of TbHKT1, wheat HKT1 (TaHKT2;1), and Enterococcus hirae KtrB.
Functional characterization of TbHKT1 in heterologous expression systems.
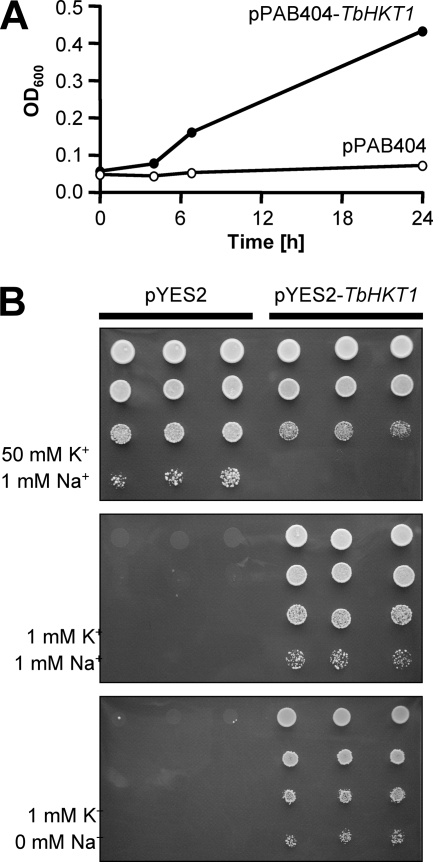
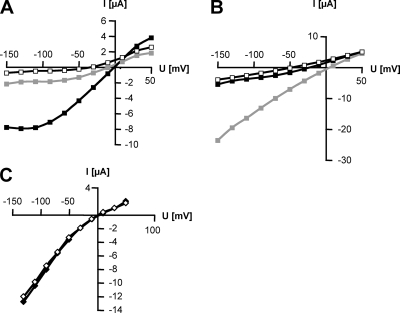
TbHKT1 was functionally expressed in E. coli, S. cerevisiae, and in Xenopus laevis oocytes. For expression in E. coli, we used the potassium transport-defective quadruple mutant L2003, which lacks the Trk/HKT superfamily genes TrkG and TrkH, the Kup gene TrkD, and the ATPase Kdp (46). LB2003 cells need ≥20 mM K+ in the medium to grow. When TbHKT1 was expressed in E. coli LB2003, the cells grew at K+ concentrations as low as 2 mM (Fig. 4A). Similar results were obtained when TbHKT1 was expressed, under the control of a Gal1 promoter, in S. cerevisiae strain CY162, a K+ uptake-defective double mutant that lacks the Trk/HKT genes Trk1 and Trk2 (20) and is unable to grow on potassium concentration below 20 mM. Expression of TbHKT1 allowed the CY162 mutant to grow on 1 mM KCl while control transformants with the empty vector required at least 20 mM KCl for growth (Fig. 4B). As some of the plant HKT proteins function as K+:Na+ symporters (42), TbHKT1-mediated K+ transport was tested for Na+ dependency. Yeast CY162 mutants cells were still able to grow on 1 mM KCl in the absence of Na+ (Fig. 4B), indicating that TbHKT1 is not a K+:Na+ symporter. To better address the substrate specificity of TbHKT1, the gene was expressed in Xenopus oocytes. A voltage ramp from −150 to 50 mV produced currents only in the presence of external K+; when K+ was substituted with Na+, only small currents were recorded (Fig. 5A). Arabidopsis AtHKT1 (51) was used as a positive control for Na+ transport (Fig. 5B). At 120 mM external K+, the currents mediated by TbHKT1 were independent of Na+ (Fig. 5C). Thus, based on the heterologous expression in bacteria, yeast, and oocytes, TbHKT1 is a Na+-independent K+ transporter. It is interesting to note that in the only multicellular eukaryotes known to possess Trk/HKT genes, the vascular plants, the majority of the transporters function in Na+ homeostasis (39). Mediating root-to-shoot transport of Na+, they are important for salt tolerance and—with a few exceptions (42)—affect K+ homeostasis only indirectly (21). The function of TbHKT1 in trypanosomes remains to be elucidated.
Fig. 4.
TbHKT1 suppresses K+ uptake deficiency in yeast and in E. coli. (A) The plasmid containing TbHKT1 allows the E. coli K+ uptake-deficient quadruple mutant LB2003 to grow in medium containing 2 mM K+; the empty plasmid does not. (B) Expression of TbHKT1 in the S. cerevisiae Δtrk1 Δtrk2 double null mutant CY162 permits growth on 1 mM K+. The effect is not dependent on Na+. Cells were spotted in triplicates, in a dilution series from optical density at 600 nm (OD600) of 1 to OD600 of 10−3.
Fig. 5.
TbHKT1 mediates K+ transport in Xenopus oocytes. Currents recorded as a function of applied voltage with oocytes expressing TbHKT1 (A and C) or AtHKT1 (B), in the presence of 100 mM K+ (black squares), 100 mM Na+ (gray squares), or 100 mM Tris (white squares) in the bath solution. Control oocytes injected with water did not exhibit any currents (data not shown). (C) In the presence of 120 mM K+ in the bath solution, the absence (white diamonds) or presence (black diamonds) of 1 mM Na+ did not affect the currents.
Functional characterization of TbHKT1 in trypanosomes.
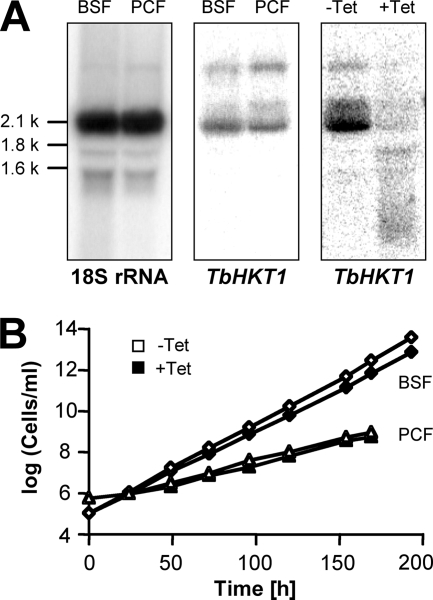
TbHKT1 is expressed in both procyclic and bloodstream-form T. b. brucei trypanosomes (Fig. 6A), albeit at low levels as the Northern blot signals were only obtained with 32P-radiolabeled probes, not with digoxigenin-labeled probes (not shown). Furthermore, in situ tagging (38) of TbHKT1 with a hemagglutinin tag did not yield an immunofluorescence signal above background, neither in bloodstream-form nor in procyclic trypanosomes (not shown). To investigate the function of TbHKT1 in trypanosomes, the expression of TbHKT1 was downregulated by RNAi-mediated gene silencing. Bloodstream-form T. b. brucei NYSM and procyclic-form T. b. brucei 29-13 were stably transformed with a stem-loop construct of a TbHKT1 fragment under the control of the Tet operator. Forty-eight hours after addition of tetracycline (1 mg/ml) to the medium, the TbHKT1 mRNA steady-state levels were reduced substantially in procyclic (Fig. 6A) as well as in bloodstream-form (not shown) trypanosomes. However, the TbHKT1 knockdown trypanosomes did not show any growth phenotype when cultivated in standard medium plus tetracycline over 10 days (Fig. 6B), indicating that expression of TbHKT1 is not required under these conditions or that residual TbHKT1 expression after RNAi was sufficient to maintain viability. When the K+ concentration in the medium was lowered from 5 mM to 0.5 mM, the trypanosomes (bloodstream forms) proliferated more slowly; but again, there was no significant difference in growth rate between TbHKT1-RNAi cells incubated with (population-doubling time of 27.3 h) or without (population-doubling time of 24.6 h) tetracycline. We were not able, after 10 independent attempts to disrupt the genes with antibiotic resistance markers, to create homozygous tbhkt1−/− knockout trypanosomes. Thus, the question of whether, and under which conditions, TbHKT1 is essential for T. brucei remains unresolved.
Fig. 6.
Expression and silencing of TbHKT1 in T. brucei. (A) Northern blot with total RNA isolated from cultured T. b. brucei bloodstream forms (BSF) and procyclic forms (PCF). A probe complementary to the 18S rRNA was used as a loading control (left panel). T. b. brucei RNAi cells that express double-stranded TbHKT1 stem-loop constructs under the control of the Tet operator downregulate TbHKT1 expression in the presence of tetracycline (right panel). (B) Growth curves with bloodstream-form (diamonds) and procyclic (triangles) T. b. brucei TbHKT1-RNAi cells; addition of tetracycline (1 mg/ml) to the medium does not impair growth.
Repeated loss of Trk/HKT genes from eukaryotes.
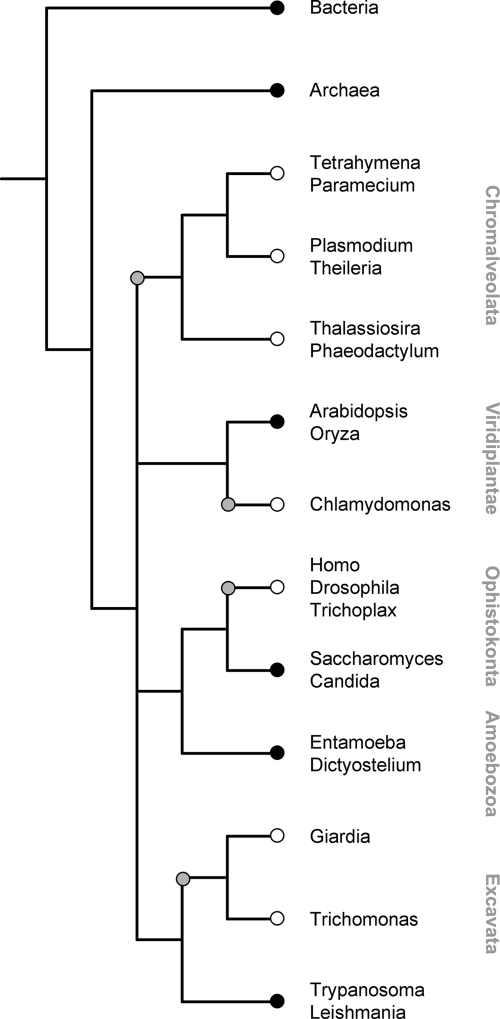
A number of genomes from endoparasitic protozoa have been fully sequenced. Besides the trypanosomatids (Table 2), we screened the predicted proteomes of Plasmodium falciparum, Theileria parva, Entamoeba histolytica, Giardia duodenalis, and Trichomonas vaginalis for the presence of Trk/HKT proteins with the same profile that was used to identify TbHKT1. Additional representatives of the major eukaryote groups were included as well: the ciliates Tetrahymena thermophyla and Paramecium tetraurelia, the diatoms Thalassiosira pseudonana and Phaeodactylum tricornutum, the amoeba Dictyostelium discoideum, and Trichoplax adhaerens as an additional metazoan. No complete genomes are available yet from the rhizaria (foraminifers, radiolarians, etc.). When the presence of Trk/HKT genes is overlaid on the phylogeny of the analyzed organisms, the emerging picture is enigmatic at first sight since the distribution of Trk/HKT genes among eukaryotes is not coherent (Fig. 7). While the genes occur in kinetoplastids, amoebae, fungi, and vascular plants, they are absent from Chlamydomonas (53), Giardia, and Trichomonas, as well as from all available chromalveolate and metazoan genomes. However, the broad occurrence of Trk/HKT genes in bacteria and archaea indicates that the primordial eukaryotes possessed Trk/HKT-type K+ transporters. Thus, the parsimonial interpretation of Fig. 7 is multiple independent gene loss, at least four times among the major eukaryote lineages: within the chromalveolates, the green plants, the ophistokonts, and the excavates (gray circles in Fig. 7). Apparently, HKT/Trk transporters tend to become redundant in the course of evolution of the mechanisms for K+ uptake and homeostasis. The fact that the trypanosomatids, in contrast to all other excavates analyzed, have retained an HKT orthologue suggests that these K+ transporters fulfill an indispensable function at some point in the life cycle of a trypanosomatid parasite.
Fig. 7.
Repeated loss of Trk/HKT genes in the major eukaryote lineages. Schematic phylogeny of fully sequenced eukaryotes that possess (black circles) or lack (white circles) genes of the Trk/HKT superfamily. The gray circles indicate presumed loss of Trk/HKT genes.
ACKNOWLEDGMENTS
We thank Gaby Schumann-Burkard for help with FACS, Evert Bakker for the E. coli strain LB2003 and plasmid pPAB404, George Cross for the T. b. brucei NYSM line, and Rick Gaber for S. cerevisiae CY162.
This work was supported by the Swiss National Science Foundation.
Footnotes
Published ahead of print on 26 February 2010.
REFERENCES
- 1.Allemann N., Schneider A. 2000. ATP production in isolated mitochondria of procyclic Trypanosoma brucei. Mol. Biochem. Parasitol. 111:87–94 [DOI] [PubMed] [Google Scholar]
- 2.Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403–410 [DOI] [PubMed] [Google Scholar]
- 3.Barrett M. P., Boykin D. W., Brun R., Tidwell R. R. 2007. Human African trypanosomiasis: pharmacological re-engagement with a neglected disease. Br. J. Pharmacol. 152:1155–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barrett M. P., Tetaud E., Seyfang A., Bringaud F., Baltz T. 1998. Trypanosome glucose transporters. Mol. Biochem. Parasitol. 91:195–205 [DOI] [PubMed] [Google Scholar]
- 5.Benito B., Garciadeblas B., Rodriguez-Navarro A. 2002. Potassium- or sodium-efflux ATPase, a key enzyme in the evolution of fungi. Microbiology 148:933–941 [DOI] [PubMed] [Google Scholar]
- 6.Berriman M., Ghedin E., Hertz-Fowler C., Blandin G., Renauld H., Bartholomeu D. C., Lennard N. J., Caler E., Hamlin N. E., Haas B., Bohme U., Hannick L., Aslett M. A., Shallom J., Marcello L., Hou L., Wickstead B., Alsmark U. C., Arrowsmith C., Atkin R. J., Barron A. J., Bringaud F., Brooks K., Carrington M., Cherevach I., Chillingworth T. J., Churcher C., Clark L. N., Corton C. H., Cronin A., Davies R. M., Doggett J., Djikeng A., Feldblyum T., Field M. C., Fraser A., Goodhead I., Hance Z., Harper D., Harris B. R., Hauser H., Hostetler J., Ivens A., Jagels K., Johnson D., Johnson J., Jones K., Kerhornou A. X., Koo H., Larke N., Landfear S., Larkin C., Leech V., Line A., Lord A., Macleod A., Mooney P. J., Moule S., Martin D. M., Morgan G. W., Mungall K., Norbertczak H., Ormond D., Pai G., Peacock C. S., Peterson J., Quail M. A., Rabbinowitsch E., Rajandream M. A., Reitter C., Salzberg S. L., Sanders M., Schobel S., Sharp S., Simmonds M., Simpson A. J., Tallon L., Turner C. M., Tait A., Tivey A. R., Van Aken S., Walker D., Wanless D., Wang S., White B., White O., Whitehead S., Woodward J., Wortman J., Adams M. D., Embley T. M., Gull K., Ullu E., Barry J. D., Fairlamb A. H., Opperdoes F., Barrell B. G., Donelson J. E., Hall N., Fraser C. M., Melville S. E., El-Sayed N. M. 2005. The genome of the African trypanosome Trypanosoma brucei. Science 309:416–422 [DOI] [PubMed] [Google Scholar]
- 7.Borst P., Fairlamb A. H. 1998. Surface receptors and transporters of Trypanosoma brucei. Annu. Rev. Microbiol. 52:745–778 [DOI] [PubMed] [Google Scholar]
- 8.Brun R., Blum J., Chappuis F., Burri C. 2010. Human African trypanosomiasis. Lancet 375:148–159 [DOI] [PubMed] [Google Scholar]
- 9.Brun R., Schönenberger M. 1979. Cultivation and in vivo cloning of procyclic culture forms of Trypanosoma brucei in a semi-defined medium. Acta Trop. 36:289–292 [PubMed] [Google Scholar]
- 10.Davenport R. J., Munoz-Mayor A., Jha D., Essah P. A., Rus A., Tester M. 2007. The Na+ transporter AtHKT1;1 controls retrieval of Na+ from the xylem in Arabidopsis. Plant Cell Environ. 30:497–507 [DOI] [PubMed] [Google Scholar]
- 11.Defrise-Quertain F., Fraser-L'Hostis C., Coral D., Deshusses J. 1996. Kinetic study of the plasma-membrane potential in procyclic and bloodstream forms of Trypanosoma brucei brucei using the fluorescent probe bisoxonol. Biochem. J. 314:595–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Koning H. P., Bridges D., Burchmore R. J. 2005. Purine and pyrimidine transport in pathogenic protozoa: from biology to therapy. FEMS Microbiol. Rev. 29:987–1020 [DOI] [PubMed] [Google Scholar]
- 13.Doyle D. A., Morais Cabral J., Pfuetzner R. A., Kuo A., Gulbis J. M., Cohen S. L., Chait B. T., MacKinnon R. 1998. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science 280:69–77 [DOI] [PubMed] [Google Scholar]
- 14.Durell S. R., Guy H. R. 1999. Structural models of the KtrB, TrkH, and Trk1,2 symporters based on the structure of the KcsA K(+) channel. Biophys. J. 77:789–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Durell S. R., Hao Y., Nakamura T., Bakker E. P., Guy H. R. 1999. Evolutionary relationship between K(+) channels and symporters. Biophys. J. 77:775–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eddy S. R. 1995. Multiple alignment using hidden Markov models. Proc. Int. Conf. Intell. Syst. Mol. Biol. 3:114–120 [PubMed] [Google Scholar]
- 17.Eddy S. R. 1998. Profile hidden Markov models. Bioinformatics 14:755–763 [DOI] [PubMed] [Google Scholar]
- 18.Ellekvist P., Maciel J., Mlambo G., Ricke C. H., Colding H., Klaerke D. A., Kumar N. 2008. Critical role of a K+ channel in Plasmodium berghei transmission revealed by targeted gene disruption. Proc. Natl. Acad. Sci. U. S. A. 105:6398–6402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ellekvist P., Ricke C. H., Litman T., Salanti A., Colding H., Zeuthen T., Klaerke D. A. 2004. Molecular cloning of a K(+) channel from the malaria parasite Plasmodium falciparum. Biochem. Biophys. Res. Commun. 318:477–484 [DOI] [PubMed] [Google Scholar]
- 20.Gaber R. F., Styles C. A., Fink G. R. 1988. TRK1 encodes a plasma membrane protein required for high-affinity potassium transport in Saccharomyces cerevisiae. Mol. Cell. Biol. 8:2848–2859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gierth M., Mäser P. 2007. Potassium transporters in plants—involvement in K+ acquisition, redistribution and homeostasis. FEBS Lett. 581:2348–2356 [DOI] [PubMed] [Google Scholar]
- 22.Hannaert V., Saavedra E., Duffieux F., Szikora J. P., Rigden D. J., Michels P. A., Opperdoes F. R. 2003. Plant-like traits associated with metabolism of Trypanosoma parasites. Proc. Natl. Acad. Sci. U. S. A. 100:1067–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harms C., Domoto Y., Celik C., Rahe E., Stumpe S., Schmid R., Nakamura T., Bakker E. P. 2001. Identification of the ABC protein SapD as the subunit that confers ATP dependence to the K+-uptake systems Trk(H) and Trk(G) from Escherichia coli K-12. Microbiology 147:2991–3003 [DOI] [PubMed] [Google Scholar]
- 24.Hirumi H., Hirumi K. 1989. Continuous cultivation of Trypanosoma brucei blood stream forms in a medium containing a low concentration of serum protein without feeder cell layers. J. Parasitol. 75:985–989 [PubMed] [Google Scholar]
- 25.Huson D. H., Richter D. C., Rausch C., Dezulian T., Franz M., Rupp R. 2007. Dendroscope: an interactive viewer for large phylogenetic trees. BMC Bioinformatics 8:460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jackson A. P. 2007. Origins of amino acid transporter loci in trypanosomatid parasites. BMC Evol. Biol. 7:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kato Y., Sakaguchi M., Mori Y., Saito K., Nakamura T., Bakker E. P., Sato Y., Goshima S., Uozumi N. 2001. Evidence in support of a four transmembrane-pore-transmembrane topology model for the Arabidopsis thaliana Na+/K+ translocating AtHKT1 protein, a member of the superfamily of K+ transporters. Proc. Natl. Acad. Sci. U. S. A. 98:6488–6493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kesteloot H., Joossens J. V. 1988. Relationship of serum sodium, potassium, calcium, and phosphorus with blood pressure. Belgian Interuniv. Res. Nutr. Health Hypertens. 12:589–593 [DOI] [PubMed] [Google Scholar]
- 29.Krogh A., Larsson B., von Heijne G., Sonnhammer E. L. 2001. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J. Mol. Biol. 305:567–580 [DOI] [PubMed] [Google Scholar]
- 30.Landfear S. M. 2008. Drugs and transporters in kinetoplastid protozoa. Adv. Exp. Med. Biol. 625:22–32 [DOI] [PubMed] [Google Scholar]
- 31.Legros D., Ollivier G., Gastellu-Etchegorry M., Paquet C., Burri C., Jannin J., Buscher P. 2002. Treatment of human African trypanosomiasis—present situation and needs for research and development. Lancet Infect. Dis. 2:437–440 [DOI] [PubMed] [Google Scholar]
- 32.Liu W., Fairbairn D. J., Reid R. J., Schachtman D. P. 2001. Characterization of two HKT1 homologues from Eucalyptus camaldulensis that display intrinsic osmosensing capability. Plant Physiol. 127:283–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mäser P., Eckelman B., Vaidyanathan R., Horie T., Fairbairn D. J., Kubo M., Yamagami M., Yamaguchi K., Nishimura M., Uozumi N., Robertson W., Sussman M. R., Schroeder J. I. 2002. Altered shoot/root Na+ distribution and bifurcating salt sensitivity in Arabidopsis by genetic disruption of the Na+ transporter AtHKT1. FEBS Lett. 531:157–161 [DOI] [PubMed] [Google Scholar]
- 34.Mäser P., Hosoo Y., Goshima S., Horie T., Eckelman B., Yamada K., Yoshida K., Bakker E. P., Shinmyo A., Oiki S., Schroeder J. I., Uozumi N. 2002. Glycine residues in potassium channel-like selectivity filters determine potassium selectivity in four-loop-per-subunit HKT transporters from plants. Proc. Natl. Acad. Sci. U. S. A. 99:6428–6433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mäser P., Lüscher A., Kaminsky R. 2003. Drug transport and drug resistance in African trypanosomes. Drug Resist. Updat. 6:281–290 [DOI] [PubMed] [Google Scholar]
- 36.Nakamura T., Yamamuro N., Stumpe S., Unemoto T., Bakker E. P. 1998. Cloning of the trkAH gene cluster and characterization of the Trk K(+)-uptake system of Vibrio alginolyticus. Microbiology 144:2281–2289 [DOI] [PubMed] [Google Scholar]
- 37.Nolan D. P., Voorheis H. P. 2000. Factors that determine the plasma-membrane potential in bloodstream forms of Trypanosoma brucei. Eur. J. Biochem. 267:4615–4623 [DOI] [PubMed] [Google Scholar]
- 38.Oberholzer M., Morand S., Kunz S., Seebeck T. 2006. A vector series for rapid PCR-mediated C-terminal in situ tagging of Trypanosoma brucei genes. Mol. Biochem. Parasitol. 145:117–120 [DOI] [PubMed] [Google Scholar]
- 39.Platten J. D., Cotsaftis O., Berthomieu P., Bohnert H., Davenport R. J., Fairbairn D. J., Horie T., Leigh R. A., Lin H. X., Luan S., Maser P., Pantoja O., Rodriguez-Navarro A., Schachtman D. P., Schroeder J. I., Sentenac H., Uozumi N., Very A. A., Zhu J. K., Dennis E. S., Tester M. 2006. Nomenclature for HKT transporters, key determinants of plant salinity tolerance. Trends Plant Sci. 11:372–374 [DOI] [PubMed] [Google Scholar]
- 40.Räz B., Iten M., Grether-Bühler Y., Kaminsky R., Brun R. 1997. The Alamar Blue assay to determine drug sensitivity of African trypanosomes in vitro. Acta Trop. 68:139–147 [DOI] [PubMed] [Google Scholar]
- 41.Rodriguez-Navarro A., Ramos J. 1984. Dual system for potassium transport in Saccharomyces cerevisiae. J. Bacteriol. 159:940–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rubio F., Gassmann W., Schroeder J. I. 1995. Sodium-driven potassium uptake by the plant potassium transporter HKT1 and mutations conferring salt tolerance. Science 270:1660–1663 [DOI] [PubMed] [Google Scholar]
- 43.Saier M. H., Jr., Tran C. V., Barabote R. D. 2006. TCDB: the Transporter Classification Database for membrane transport protein analyses and information. Nucleic Acids Res. 34:D181–D186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schachtman D. P., Schroeder J. I. 1994. Structure and transport mechanism of a high-affinity potassium uptake transporter from higher plants. Nature 370:655–658 [DOI] [PubMed] [Google Scholar]
- 45.Stiles J. K., Kucerova Z., Sarfo B., Meade C. A., Thompson W., Shah P., Xue L., Meade J. C. 2003. Identification of surface-membrane P-type ATPases resembling fungal K(+)- and Na(+)-ATPases, in Trypanosoma brucei, Trypanosoma cruzi and Leishmania donovani. Ann. Trop. Med. Parasitol. 97:351–366 [DOI] [PubMed] [Google Scholar]
- 46.Stumpe S., Bakker E. P. 1997. Requirement of a large K+-uptake capacity and of extracytoplasmic protease activity for protamine resistance of Escherichia coli. Arch. Microbiol. 167:126–136 [PubMed] [Google Scholar]
- 47.Sunarpi , Horie T., Motoda J., Kubo M., Yang H., Yoda K., Horie R., Chan W. Y., Leung H. Y., Hattori K., Konomi M., Osumi M., Yamagami M., Schroeder J. I., Uozumi N. 2005. Enhanced salt tolerance mediated by AtHKT1 transporter-induced Na unloading from xylem vessels to xylem parenchyma cells. Plant J. 44:928–938 [DOI] [PubMed] [Google Scholar]
- 48.Tholema N., Bakker E. P., Suzuki A., Nakamura T. 1999. Change to alanine of one out of four selectivity filter glycines in KtrB causes a two orders of magnitude decrease in the affinities for both K+ and Na+ of the Na+ dependent K+ uptake system KtrAB from Vibrio alginolyticus. FEBS Lett. 450:217–220 [DOI] [PubMed] [Google Scholar]
- 49.Tholema N., Vor der Bruggen M., Maser P., Nakamura T., Schroeder J. I., Kobayashi H., Uozumi N., Bakker E. P. 2005. All four putative selectivity filter glycine residues in KtrB are essential for high affinity and selective K+ uptake by the KtrAB system from Vibrio alginolyticus. J. Biol. Chem. 280:41146–41154 [DOI] [PubMed] [Google Scholar]
- 50.Thompson J. D., Higgins D. G., Gibson T. J. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673–4680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Uozumi N., Kim E. J., Rubio F., Yamaguchi T., Muto S., Tsuboi A., Bakker E. P., Nakamura T., Schroeder J. I. 2000. The Arabidopsis HKT1 gene homolog mediates inward Na+ currents in Xenopus laevis oocytes and Na(+) uptake in Saccharomyces cerevisiae. Plant Physiol. 122:1249–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Waller K. L., McBride S. M., Kim K., McDonald T. V. 2008. Characterization of two putative potassium channels in Plasmodium falciparum. Malar. J. 7:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ward J. M., Maser P., Schroeder J. I. 2009. Plant ion channels: gene families, physiology, and functional genomics analyses. Annu. Rev. Physiol. 71:59–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wirtz E., Leal S., Ochatt C., Cross G. A. 1999. A tightly regulated inducible expression system for conditional gene knock-outs and dominant-negative genetics in Trypanosoma brucei. Mol. Biochem. Parasitol. 99:89–101 [DOI] [PubMed] [Google Scholar]
- 55.Zeng G. F., Pypaert M., Slayman C. L. 2004. Epitope tagging of the yeast K(+) carrier Trk2p demonstrates folding that is consistent with a channel-like structure. J. Biol. Chem. 279:3003–3013 [DOI] [PubMed] [Google Scholar]
- 56.Zilberstein D. 1993. Transport of nutrients and ions across membranes of trypanosomatid parasites. Adv. Parasitol. 32:261–291 [DOI] [PubMed] [Google Scholar]