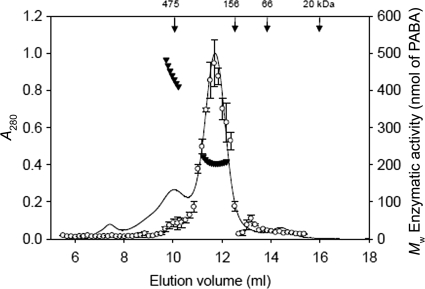
FIG. 5.
Determination of the oligomeric state of PGH from SEC-UV/LS/RI analyses and its correlation with enzymatic activity. Ni-NTA agarose-purified PGH (0.8 mg) was analyzed on a Superdex 200 HR 10/300 SEC column as described in Materials and Methods. Aliquots from fractions (5 μl) were incubated for 15 min using 200 mM PABA-GLU in the spectrophotometric assay, as described in Materials and Methods. The vertical arrows indicate the elution positions of molecular mass markers. Weight-averaged molecular weights (MW) ▾ and PGH activity ○ are plotted; the error bars represent standard deviations. The line corresponds to the absorbance (280 nM) trace of the protein eluting from the SEC column. Molecular weights were recorded every 5 μl across the elution profile (for clarity, only every 10th measurement is plotted). The average for the major eluting peak, from three independent SEC-UV/LS/RI analyses, was 206,000 ± 2,000 g/mol.