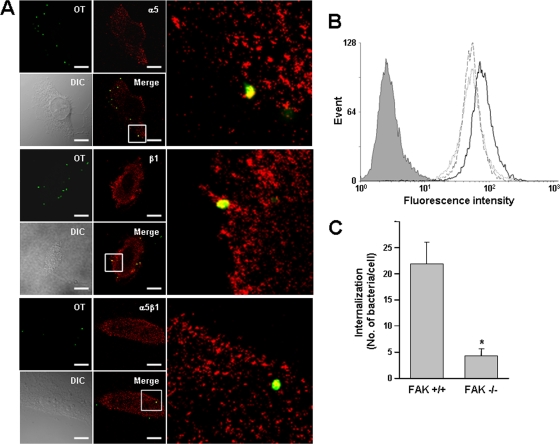
FIG. 1.
Colocalization of O. tsutsugamushi with cellular integrin α5β1 and involvement of integrin signaling in bacterial entry. (A) Immunofluorescence confocal microscopy of infected HeLa cells showed that O. tsutsugamushi (green) colocalizes with integrin α5 and β1 subunits, as well as the integrin α5β1 heterodimeric complexes (red), at 10 min postinfection. The boxes indicate the regions of the merged images that are shown at a higher magnification on the right. OT, Orientia tsutsugamushi; DIC, differential interference contrast. Bars, 5 μm. (B) Bacterial infectivity as measured by flow cytometry for control RIE1 cells (dotted line) and recombinant cells overexpressing human integrin α5 (RIE1/α5 cells) (solid line) or mutant integrin α5 lacking the cytoplasmic tail (RIE1/α5tl cells) (dashed line). Intracellular bacteria were stained with anti-TSA56 antibody and Alexa Fluor 488-conjugated secondary antibody. The mean fluorescence intensity (MFI) and standard deviation for each group are described in the text. The gray histogram indicates the results for the isotype control. (C) Effect of FAK on bacterial infectivity. Cellular invasion by O. tsutsugamushi was evaluated using wild-type (FAK+/+) or FAK-null (FAK−/−) embryonic fibroblasts by counting the internalized bacteria using confocal microscopy. The average number of internalized bacteria per cell was determined using 100 randomly selected cells. The bars and error bars indicate the means and standard deviations of triplicate experiments. *, P < 0.01.