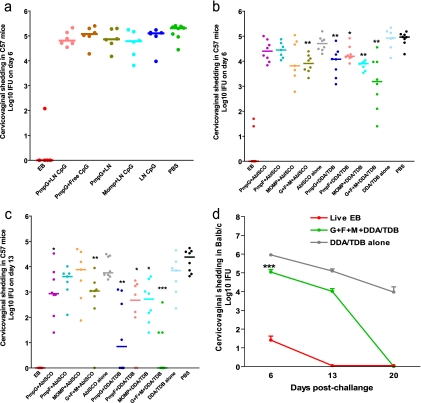
FIG. 1.
Vaccine-elicited protection against Chlamydia genital tract infection. Mice were intravaginally challenged with 1,500 IFU of live C. muridarum after immunization with a variety of vaccine formulations. Cervicovaginal washes were taken at selected dates after infection, and bacterial titers were measured on HeLa 229 cells. *, **, and *** indicate P values of <0.05, <0.01, and <0.001, respectively, in comparison to the corresponding adjuvant-alone group. (a) Failure to induce protection after vaccination with PmpG-1 or MOMP protein formulated with CpG-ODN. (b and c) Resistance to Chlamydia infection in C57BL/6 (C57) mice immunized with PmpG-1, PmpE/F-2, and MOMP proteins or a combination of them formulated with adjuvant AbISCO-100 or DDA/TDB. Cervicovaginal washes were taken at day 6 (b) and day 13 (c) after infection. (d) Resistance to Chlamydia infection in BALB/c mice (n = 8) immunized with the combination of PmpG-1, PmpE/F-2, and MOMP proteins formulated with the adjuvant DDA/TDB.