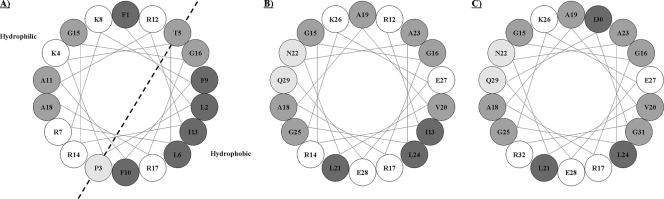
FIG. 3.
Helical wheel projections of N-terminal regions of Defb14-1CV and inactive/active derivatives. Schematic helical wheel projections of Defb14-1CV (residues 1 to 18) (A), Defb14-1CVΔ(1-11) (residues 12 to 29) (B), and Defb14-1CVΔ(1-14) (residues 15 to 32) (C). Charged residues are denoted by white circles. Increasing hydrophobicity is denoted by increasing shading. The positions of the side chains are shown along a “regular” α-helix. For a right-handed helix with 3.6 residues per turn, rotation is clockwise as the polypeptide chain is followed from N to C, and the 5th residue ends up in a position exactly 40° clockwise relative to that of residue 1.