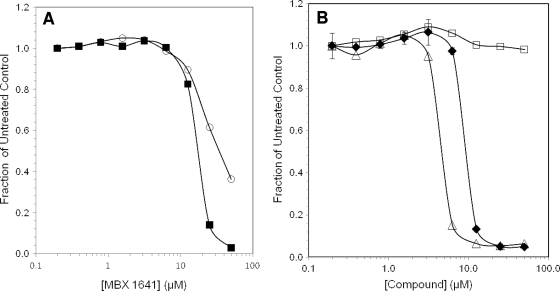
FIG. 4.
Inhibition of T3SS-mediated secretion of effector-β-lactamase fusion proteins. (A) Cells growing under T3SS-inducing conditions were treated for 3 h with MBX 1641, and secreted β-lactamase activity was measured by the cleavage of nitrocefin as the ΔA490/min. The rate of nitrocefin cleavage as a fraction of that of the untreated control is plotted versus the compound concentration. Bacterial species and effector βLA fusions were P. aeruginosa ExoS′-βLA (▪) and Y. pestis YopE-βLA (○). (B) Effects of MBX 1641 and its R- and S-enantiomers on ExoS′-βLA secretion from P. aeruginosa. Concentration dependence for MBX 1641 and its two stereo isomers, MBX 1684 and MBX 1686, were determined by the rate of nitrocefin cleavage by secreted ExoS′-βLA and calculated as the fraction of cleavage in the absence of inhibitor. Racemic mixture MBX 1641 (⧫), R-enantiomer MBX 1684 (Δ), and S-entantiomer MBX 1686 (□) are indicated.