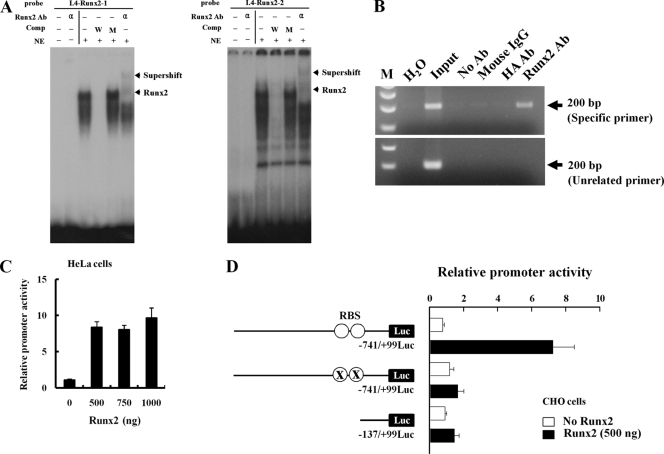
FIG. 3.
Runx2 binds directly to aromatase promoter I.4. (A) EMSA was conducted with probes containing the two wild-type Runx2 motifs. 32P-labeled wild-type oligonucleotides were incubated with nuclear extracts from HOS cells in the presence of a 100-fold molar excess of unlabeled specific oligonucleotides or unlabeled mutant oligonucleotides or in the presence of anti-Runx2 antibody (α). The bottom arrows indicate the positions of the major nuclear protein-DNA complexes, and the top arrows indicate Runx2-supershifted complexes. (B) ChIP analysis was performed with formaldehyde-cross-linked chromatin isolated from HOS cells and antibody against Runx2. PCR amplification was carried out as described in Materials and Methods for a total of 28 cycles, and amplified fragments were analyzed by 2% agarose gel electrophoresis. (C) HeLa cells were cotransfected with the promoter I.4 reporter construct and Runx2 expression vector together with a β-galactosidase (β-Gal) internal control vector. The results were normalized to the protein concentration and β-Gal activity to account for transfection efficiency. (D) Runx2 did not stimulate Luc activity from a promoter with a mutant Runx2 binding site (RBS) or 0.14-kb aromatase promoter I.4 in CHO cells. Data represent the means ± SD from three independent experiments. Ab, antibody; Comp, competitor; NE, nuclear extract; W, wild-type oligonucleotide; M, mutant oligonucleotide; ○, RBS (TGTGGT and ACCACA); X, RBS mutation (TGTacT and AgtACA).