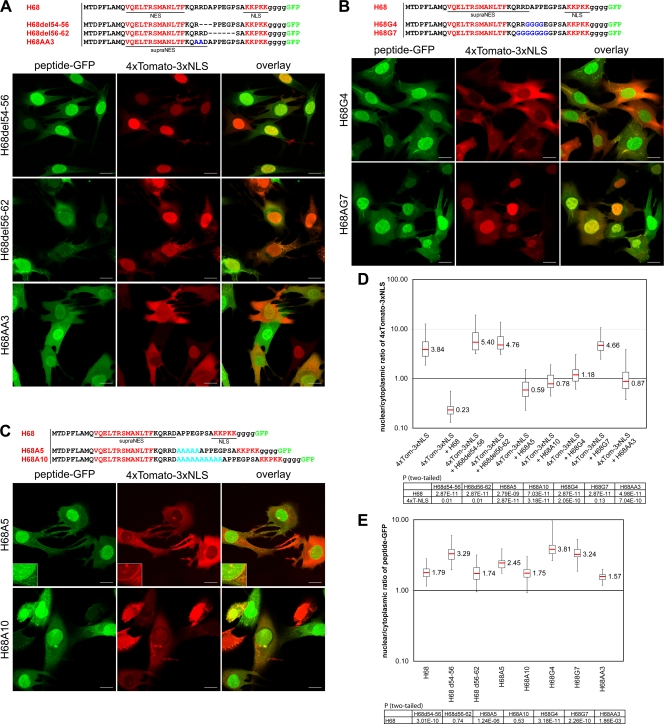
FIG. 6.
Mutations in the NES- and NLS-connecting linker affect the ability of H68 peptide to inhibit nuclear import. In all of the experiments presented, BHK-21 cells were coinfected with packaged VEEV replicons encoding mutant-peptide-GFP fusions and 4×Tomato-3×NLS. The cells were fixed at 4 h postinfection, and the distribution of fluorescent proteins was analyzed using a Leica SP1 confocal microscope. (A) (Top) Amino acid sequences of the wild-type H68 peptide and the deletion or substitution mutants. The positions of the deletions are indicated by dashed lines. (Bottom) The deletions of 3 or 5 aa in the linker peptide completely abolished nuclear import inhibition by mutant-peptide-GFP fusions. H68del54-56 accumulated more efficiently in the nuclei than H68-GFP (compare Fig. 1A and panel E). (B) (Top) Amino acid sequences of the wild-type H68 peptide and the designed mutants. Four or 7 amino acids of the linker were replaced with glycines, which are highlighted in blue. (Bottom) Substitution of 4 aa (H68G4) partially restored the nuclear import of 4×Tomato-3×NLS. Substitution of 7 amino acids (H68G7) completely abolished the ability of the mutant peptide (H68G7) to inhibit nuclear import. (C) (Top) Amino acid sequences of the wild-type H68 peptide and the insertion mutants. Five- and 10-aa-long linkers were inserted between the supra-NES and NLS. (Bottom) In the presence of both mutated GFP fusions, nuclear import of the 4×Tomato-3×NLS reporter was partially restored. The mutant peptide with the longer linker had less inhibitory effect on nuclear import. The enlarged insets demonstrate the accumulation of 4×Tomato-3×NLS and H68A5-GFP at the nuclear rim. (D) The box plot demonstrates the nuclear/cytoplasmic distribution of 4×Tomato-3×NLS expressed alone or in the presence of a mutant peptide-GFP. (E) The box plot demonstrates the nuclear/cytoplasmic distribution of H68-GFP and mutant proteins. The P values were calculated using the Mann-Whitney test (n = 30 for all experiments). Scale bars, 20 μm.