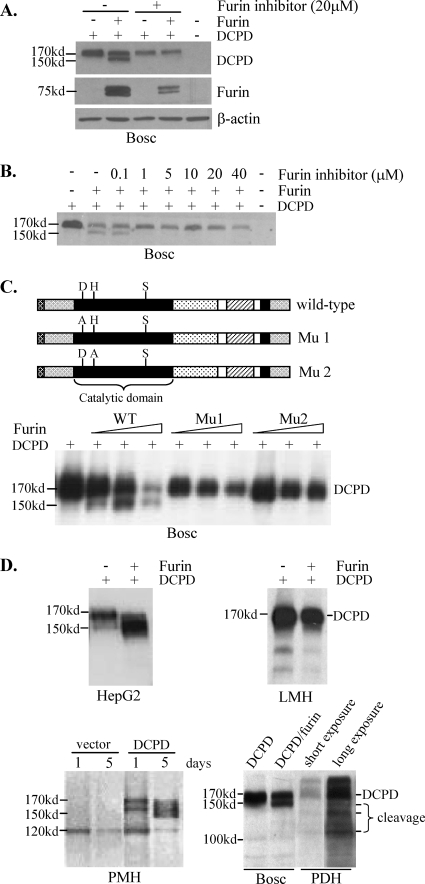
FIG. 2.
DCPD cleavage by chicken furin or endogenous furin-like PC. (A) Ability of chicken furin to cleave DCPD in Bosc cells. Cells grown in 6-well plates were cotransfected with 0.5 μg each of DCPD cDNA and the pcDNA3.1/Zeo− vector or chicken furin cDNA. The furin inhibitor (decanoyl-RVKR-chloromethylketone) was added immediately after transfection. Cells were harvested 2 days later for Western blot analysis of DCPD, chicken furin, and β-actin. (B) Minimum concentration of the furin inhibitor required to block DCPD cleavage. (C) Critical role of catalytic sites of chicken furin for DCPD processing. D197, H235, and S409 in the catalytic domain (black box) form the catalytic triad. Mu1 and Mu2 contain D197A and H235A substitutions, respectively. Bosc cells in 6-well plates were either transfected with 1 μg of DCPD cDNA alone or cotransfected with DCPD and chicken furin cDNA (wild type [WT] or mutant) at a 1:16, 1:8, or 1:4 ratio, with the total amount of DNA kept at 1 μg. Cells were harvested 2 days later for DCPD detection. (D) DCPD cleavage in different cell types in the presence or absence of exogenous furin. DCPD and chicken furin cDNAs were delivered to HepG2, LMH, and Bosc cells by transient transfection and to primary mouse hepatocytes (PMH) by the adenovirus vector. The endogenous DCPD pattern in PDH was included for comparison (lower right).