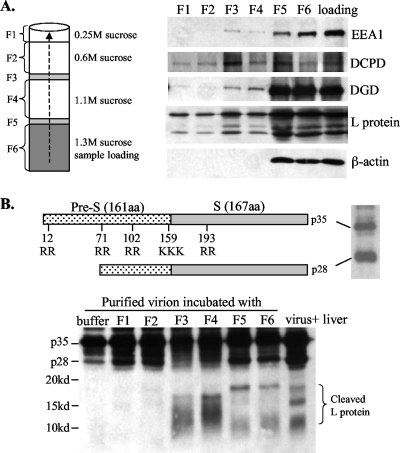
FIG. 3.
(A) Colocalization of DCPD, DGD, and viral envelope proteins in the endosomal fraction of infected duck liver. Duck liver was homogenized and mixed with sucrose solution to achieve a final concentration of 1.3 M. The mixture was loaded into the bottom of the centrifugation tube and overlaid with three additional layers of sucrose solutions. After ultracentrifugation, six fractions were collected from the bottom (note that F3 and F5 corresponded to two narrow visible protein bands). A 5-μl aliquot from each fraction was used for Western blot analysis of DCPD, DGD, and β-actin as well as EEA1, an early endosome marker (53). The sample prior to centrifugation (loading) was analyzed in parallel. (B) Cleavage of DHBV L protein by endosomal resident proteases. Shown at the top are the schematic representations of DHBV L protein (p35), its processed form (p28), and dibasic or tribasic residues. Approximately 3 × 107 purified virus particles were incubated at 37°C for 3 h with 2 μl of endosomal fractions (F1 to F6) derived from the liver of a DHBV-free duck or just with the digestion buffer. The cleavage product was resolved by 15% PAGE and detected by Western blot analysis with a polyclonal antibody against the pre-S domain. Liver lysate from a DHBV-infected duck served as a control. Note that short electrophoresis was performed to reveal <20-kDa cleavage products.