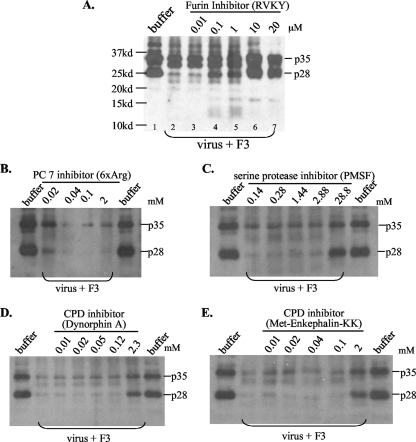
FIG. 5.
Effects of protease inhibitors on endosomal cleavage of DHBV L protein. Purified virus particles (3 × 107) were incubated with 2 to 3 μl of endosome fraction F3 in 20 mM HEPES, pH 5.5, 5 mM MgCl2, and 3 mM CaCl2, with various protease inhibitors, at 37°C for 3 h. Proteins were separated by 12% PAGE. (A) Decanoyl-RVKR-chloromethylketone as the furin inhibitor. Note that p28 was more accessible to cleavage than p35. (B) H-RRRRRR-OH (6×Arg) as the PC7 inhibitor. (C) PMSF as a broad serine protease inhibitor. (D and E) Dynorphin A and Met-enkephalin-KK as carboxypeptidase (CPD) inhibitors. Note that only the furin inhibitor significantly suppressed cleavage of DHBV L protein at the low concentrations of 0.1 to 10 μM. While cleavage products of <20 kDa are visible in panel A, for the other panels a longer gel electrophoresis was taken and cleavage of L protein was judged by decreased intensities of p35 and p28.