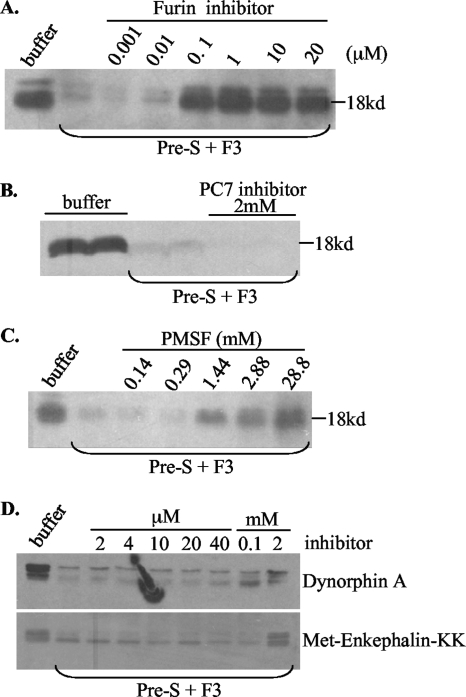
FIG. 6.
Effects of protease inhibitors on cleavage of the pre-S domain by duck liver endosome. The entire 161-aa pre-S domain of the L protein was expressed as a GST fusion protein, purified using glutathione Sepharose beads, and separated from the GST tag by thrombin digestion. The pre-S domain thus released was incubated with the endosome fraction F3 at 37°C for 3 h in 20 mM HEPES, pH 5.5, 5 mM MgCl2, and 3 mM CaCl2, with various protease inhibitors. The 18-kDa pre-S domain was revealed by Western blot analysis after electrophoresis with a 12% gel. (A) Furin inhibitor. (B) PC7 inhibitor. (C) Broad serine protease inhibitor. (D) Two carboxypeptidase inhibitors, dynorphin A (top) and Met-enkephalin-KK (bottom). Note that cleavage of the 18-kDa pre-S peptide was judged by the intensity of the 18-kDa protein that survived digestion, because no cleavage products of <18 kDa could be detected.