FIG. 5.
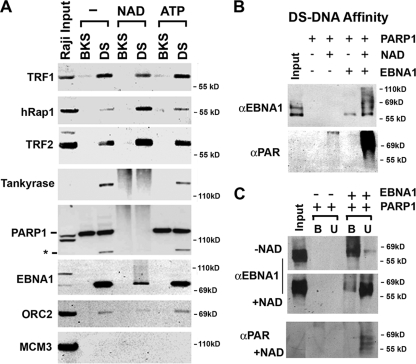
Poly(ADP-ribosyl)ation alters protein binding to the DS element. (A) Raji nuclear extracts were subjected to affinity chromatography with biotinylated DS element or control BKS DNA. Purified complexes coupled with beads were then incubated with buffer control, NAD (1 mM), or ATP (1 mM). Proteins were then eluted and assayed by SDS-PAGE and Western blotting with antibodies for TRF1, hRap1, TRF2, tankyrase 1, PARP1, EBNA1, ORC2, or MCM3. The asterisk indicates a cleaved form of PARP1 that binds specifically to the DS site. (B) Purified recombinant PARP1 and EBNA1 proteins were bound to biotinylated DS element DNA immobilized on magnetic beads and then incubated with or without NAD+ (1 mM). Proteins were then eluted and analyzed by SDS-PAGE and Western blotting with anti-EBNA1 or anti-PAR antibody. (C) DS element DNA affinity-bound PARP1 and EBNA1 were incubated without or with NAD and then separated by centrifugation for bound (B) and unbound (U) proteins. Samples were then analyzed by SDS-PAGE and Western blotting with antibodies to EBNA1 (top panels) or anti-PAR (lower panel).