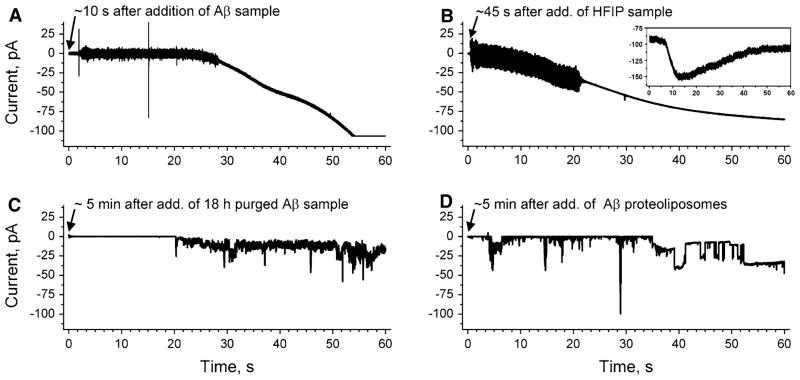
Fig. 1.
Comparison of ion flux across lipid bilayers upon exposure to samples of amyloid-β peptides (Aβ), which were prepared by two different protocols (final Aβ concentration 1 μM). a Gradual increase in transmembrane ion flux upon addition of an Aβ sample that was prepared by purging the solvent hexafluoroisopropanol (HFIP) for 10 min (Kayed et al. 2004; Sokolov et al. 2004). The “noisy” part of the current trace is a result of stirring. b Gradual increase in transmembrane ion flux following the exact same protocol as in (a) but in the absence of Aβ. Inset shows gradual increase in ion flux across the membranes of SH-SY5Y cells upon addition of HFIP (final concentration ~6 mM). c Transmembrane ion flux upon addition of an Aβ sample prepared according to previous reports (Kayed et al. 2004; Sokolov et al. 2004) but with 18 h instead of 10 min of purging to remove HFIP. d Stepwise fluctuations in transmembrane ion flux after incorporating a proteoliposome preparation of Aβ that was prepared without using HFIP (Arispe et al. 1993b). The applied voltage was −150 mV in all recordings. Membranes were prepared from DOPC:DOPE lipids at a 1:1 (w/w) ratio by the “folding method” over a pore with a diameter of 150 μm in a Teflon film that was pretreated with 5% squalene in pentane