Abstract
Background
The objective of this article was to design, fabricate, and evaluate a novel type of glucose biosensors based on the use of atomic force microscopy to create nanoindented electrodes (NIDEs) for the selective detection of glucose.
Methods
Atomic force microscopy nanoindentation techniques were extended to covalently immobilized glucose oxidase on NIDEs via composite hydrogel membranes composed of interpenetrating networks of inherently conductive poly(3,4-ethylenedioxythiophene) tetramethacrylate grown within ultraviolet cross-linked hydroxyethylmethacrylate-based hydrogels to produce an in vitro amperometric NIDE biosensor for the long-term monitoring of glucose.
Results
The calibration curve for glucose was linear from 0.25 to 20 mM. Results showed that the NIDE glucose biosensor has a much higher detection sensitivity of 0.32 μA/mM and rapid response times (<5 seconds). There was no interference from the competing interferent (fructose) present; the only interference was from species that react with H2O2 (ascorbic acid). The linear equation was Bresponse (μA) = 0.323 [glucose] (mM) + 0.634 (μA); n = 24, r2 = 0.994.
Conclusion
Results showed that the resultant NIDE glucose biosensor increases the dynamic range, device sensitivity, and response time and has excellent detecting performance for glucose.
Keywords: amperometric; atomic force microscopy; glucose biosensor; hydrogel; nanoindented electrodes; poly(3,4-ethylene dioxythiophene)
Background
Currently, the diagnosis of diabetes employs sensitive methods for measuring blood glucose such as the utilization of enzyme glucose oxidase (GOx) in the presence of oxygen to catalyze the oxidation of β-D-glucose to D-gluconic acid and hydrogen peroxide:1–3
Typically, the electrochemical detection of the product, H2O2, in the presence of oxygen is usually accomplished by the oxidation of H2O2 at an anodic potential of 0.7 volt against an Ag/AgCl reference electrode.4 Clark and Lyons first introduced the concept of a glucose sensor in 1962, which was achieved through integration of the oxidoreductase GOx with an oxygen electrode.5 In 1967, Updike and Hick6 immobilized glucose oxidase in a polyacrylamide gel at an oxygen electrode. This pioneering work led to the progress and development of clinically useful biosensors. This biosensor technology has dominated the commercial clinical market since the 1960s. In general, most biosensors are based on electrochemical detection principles and employ enzymes as the biorecognition layer. Numerous studies on the determination of glucose have been reported, such as amperometric,7 potentiometric,8 chemiluminometric,9 spectrophotometric,10 and fluorometric methods.11 In addition, numerous materials, sensing configurations, and fabrication techniques have been reported for the construction of biosensors for glucose detection.12–21 The reason for this high demand is the escalating mortality rate and life-threatening complications associated with the disease diabetes mellitus, which accounts for about 300 million sufferers worldwide.
To date, the development of a stable, highly accurate and continuous implantable biosensor for glucose that is acceptable to diabetes patients remains a significant challenge to researchers.22 Thus far, the most promising results have been obtained with biosensors based on the amperometric detection of nascent hydrogen peroxide produced by enzymes immobilized on electrodes or the alternative, which is based on voltametrically discharged oxygen at an enzyme-modified electrode. The significance of this metabolite and its intricate relationship as gauges for chronic illness has been the subject of investigation and is still to be fully understood.15 This pandemic is characterized by a failure to maintain blood glucose at suitable levels, primarily due to pancreatic dysfunction. Gauges for chronic illness have been the subject of investigation and are still to be fully understood.23 Thus the development of advanced biosensors requires proper attention to both the biorecognition layer and the physical transducer, as well as to the coupling of these recognition and transduction events through control of the surface chemistry and coverage. Several electrode geometries have been investigated and reported for glucose sensing, such as microdiscs,4,24 interdigitated microelectrodes,25 and ultramicroelectrodes.26 In this article, atomic force microscopy (AFM) nanoindentation techniques were extended to entrap glucose oxidase enzyme within new composite materials and subsequent deposition onto design nanoindented electrodes (NIDEs) to produce in vitro amperometric biosensors for the detection of glucose. This article reports on the evaluation and analytical performance of the NIDE glucose biosensor containing a “biosmart” hydrogel formulation27 that contains a conducting polymer poly(3,4-ethylenedioxythiophene) (PEDOT) component. This method is simple, sensitive, and specific and has a fast response time. Work is being performed on various fundamental aspects of recognition and transduction events, and new permselective membrane materials and new electrode transducers are being developed and characterized.4,27,28 Attention is also focused on the electrode transducer and the biorecognition layer utilizing several lithography and surface chemistry techniques.
Research Design and Methods
Reagents
Hydroxyethyl methacrylate (HEMA), tetraethyleneglycol diacrylate (TEGDA), poly(ethylene glycol methacrylate (PEGMA), and PEDOT tetramethacrylate were purchased from Sigma-Aldrich. Hydroxyethyl methacrylate was passed through a prepacked column for removing hydroquinone, and monomethyl ether hydroquinone was purchased from Sigma-Aldrich. Glucose oxidase (30 mg, type VII-S), E.C. 1.1.3.4. from Aspergillus niger, and 150,000 U/mg solid and β-D-(+)-glucose were purchased from Sigma-Aldrich and used as received. All other chemicals were of analytical grade and used without further purification. Citrate phosphate buffer (0.1 M) with various pH values was prepared by mixing citric acid with potassium phosphate (0.1 M each component) and adjusting the pH with 0.1 M H3PO4 or NaOH. All solutions were made up with double-distilled water.
Instruments
Electrochemical measurements were performed with the Princeton Applied Research PAR 2273 advanced electro-chemical system (potentiostat/galvanostats). A conventional three-electrode setup was used. A platinum wire (counter electrode), Ag/AgCl (3 M KCl) reference electrode, and working NIDE were inserted into a modified 5- to 10-ml cell (BAS, Model MR-1194) for measurements. A magnetic stirrer provided the convective transport at 150 rpm during the amperometric measurements, and the background current was allowed to decay to a steady-state value before spiking the equilibrated β-D-glucose. Nanoindentation and topography images were obtained on an atomic force microscope (Agilent Technologies AFM, Series 5500 AFM/SPM). All experiments were conducted in ambient conditions at approximately 25°C.
Electrode Design and Construction
Planar microelectrodes (PMEs) were obtained from ABTECH Scientific. Metallization on these PMEs typically involves deposition of 10 nm of a titanium–tungsten alloy (Ti/W, adhesion layer) followed by 100 nm of gold, platinum, palladium, or silver metal via electron gun sputtering or magnetron sputtering on an electronics-grade borosilicate glass substrate. All PMEs were washed in boiling trichloroethylene, followed by boiling acetone, 3 minutes in each solvent, and then washed ultrasonically in 2-proponol followed by a distilled water rinse. Afterward, a treatment at 75°C for 30 seconds with a solution comprising a 5:1:1 volume ratio of distilled water, hydrogen peroxide (20% volume), and aqueous ammonia (0.1 M) ensued. Electrode surfaces were then rinsed ultrasonically in deionized water. The NIDE was fabricated from the cleaned PMEs in a multistage photolithographic process and nanolithographic process employing AFM nanomachining on gold PMEs. An active window was formed using photolithography on the surface of the cleaned PMEs. The PMEs possess a positive photoresist topcoat that was spin coated and hard baked. The optical photomask was aligned over the PMEs, and the resist was developed to passivate the PMEs and expose the underlying metal (active window).
Following photolithography, the Agilent AFM closed-loop scanner with PicoLith software from Molecular Imaging was used to indent nanometer-scale features29–31 in the active window by precisely nanoindenting with a Berkovitch indenter (a three-sided pyramid diamond tip), which was progressively pressed onto the sample surface with a 25-μN scale force. After reaching the maximum indentation depth, the tip was removed from the sample gradually, leaving behind an indentation on the sample surface. It is estimated that the tip radius of the nanoindenter is approximately 25 nm. An array of nanoscale indents was made on the PME (indentation spacing: approximately 3.5–12 μm from center on center), thus resulting in NIDE. All indentation measurements were conducted at an excitation frequency of 45 kHz. These nanoindents were arranged in a rectangular array. With the NIDE arrangement, a total of approximately 16,000 indents were accommodated within the 0.1-cm2 working electrode area. The NIDEs were completely chemically modified with an organosilane adhesion-promoting layer of γ-aminopropyl trimethoxysilane followed by coupling to acryloyl (polyethylene glycol) N-hydroxysuccinimide ester (molecular weight 3400; Nektar Therapeutics, Huntsville, AL). The former derivatizes the biosensor surface for direct covalent coupling to the composite hydrogel-sensing membranes, while the latter approach promotes hydrogen bonding to the composite gels.
The Sensing Layer—Composite Hydrogel Membranes
Previous manuscripts4,27,28 detailed the synthesis and characterization of composite hydrogel membranes composed of interpenetrating networks of inherently conductive polypyrrole grown within ultraviolet (UV) cross-linked HEMA-based hydrogels. Entrapment of the appropriate oxidase enzyme within these composite materials and subsequent deposition onto electrodes has produced clinically demonstrated amperometric biosensors for glucose, cholesterol, galactose, and lactate. These in vitro biosensors demonstrated excellent analytical characteristics: expanded linear response range (10-5–10-2 M), rapid response times (<60 seconds), and good reproducibility and recovery. In the current design of the in vitro biosensor, the biorecognition layer uses a previously published polymer membrane formulation4,27,28 that was modified. The pyrrole component of the monomer formulation was substituted by PEDOT. This bifunctional PEDOT derivative (PEDOT tetramethacrylate) has a UVpolymerizable methacroyl terminus and an oxidatively polymerizable ethylenedioxythiophene terminus and serves as a cross-linker between poly(HEMA) chains and ethylenedioxythiophene chains.
The monomer mixture comprising the various methacrylate components and the cross-linking agent (TEGDA) served as the “receiving” mixture for the oxidase enzyme and photoinitiator. The mixture was applied to the active electrode area of the biosensor. To provide for long-term stabilization of membrane-immobilized oxidoreductases, a polyethylene glycol methacrylate (PEGMA) monomer was added to the biosmart hydrogel formulation. This established poly(ethylene glycol)(PEG) chains, set pendant to the polymer network, in the form of network- supported polymer brushes. Poly(ethylene glycol) chains are well known to stabilize proteins, preventing their denaturation and promoting long-term bioactivity.32–34 Ultraviolet-induced polymerization of the methacrylate components followed immediately by electrochemical polymerization of the derivatized ethylenedioxythiophene functionality produced the final composite membrane. Polymerization of this derivative in a N2-satuarated 0.1 M KH2PO4/KCl solution (pH 7.0) secured the electro-active PEDOT component to the hydrogel network, thus preventing any possible in vitro leeching of the conductive polymer component from the composite membrane.
Fabrication of Nanoindented Electrode Glucose Biosensors
The NIDE glucose biosensor motif containing the hydrogel formulation described previously was demonstrated. Glucose oxidase was dissolved in ethylene glycol:water (1:1, v/v) and then introduced into the receiving monomer cocktail comprising HEMA, PEGMA, TEGDA, and PEDOT in a mole ratio of 80:5:10:5 mol%. Photoinitiator dimethoxyphenyl acetophenone (4 wt%) was then completely dissolved in the mixture, and 3 μl of the enzyme–monomer mixture was applied to the active electrode areas of a platinized NIDE. Ultraviolet irradiation (2.3 W/cm2, 366 nm, Spectrolinker XL-1000 UVcrosslinker, Spectronics Corporation, Westbury, NY) was performed for 10 min to polymerize the methacrylates followed immediately by electropolymerization in 0.4 M PEDOT solution (PBKCl, pH 7.0, 0.1 M in each component) at +850 mV (versus Ag/AgCl) to grow the PEDOT tetramethacrylate component within the cross-linked methacrylate network.
Results
Figure 1 (left) schematically depicts the NIDE device used in this work and shows the indent arrangements. The NIDEs are single electrodes formed from a single contiguous layer of electrode material with electrolyte exposures that occur through an array of nanodimensioned indents formed on the electroactive window. The geometric area of the exposed electrode is therefore divided among an array of nanodimensioned indents (approximately 456 nm diameter on average). The depth of the indents was examined via cross-sectional analyses and was on average 58 nm. The platinized NIDE glucose biosensor was evaluated as a substrate onto which the glucose oxidase composite hydrogel-containing PEDOT component was deposited to evaluate the analytical performance of the NIDE enzyme biosensor. In previous work,4 the polymer adopted as the conducting host material was polypyrrole, which has been shown to exhibit shortcomings due to its lack of stability, which can impact the performance of the polypyrrole-containing hydrogel biosensor during the long-term implantation process.
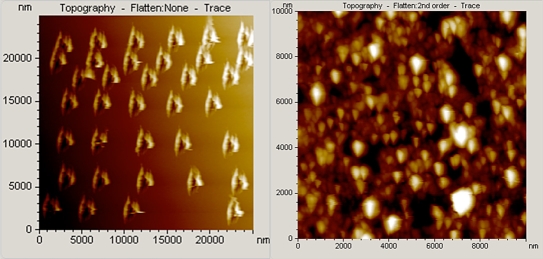
Figure 1.
Atomic force microscopy topographic images. (Left) Nanoindented arrays (25 × 25 μm2) of a clean gold (111) surface. Indents decorate the monatomic gold substrate with “cavities.” (Right) Topographic image (10 × 10 μm2) for the p(HEMA)-PEDOTPEGMA composite hydrogel prepared by electrochemical deposition at +0.850 volt for 100 seconds.
Poly(3,4-ethylenedioxythiophene) has been used for biomedical applications and has been shown to have exceptional long-term stability,35 and this highly stable material is desirable because it can endure the long-term implantation process because of its unusual environmental stability.33–37 This polymer has relatively high transparency in the oxidized state,37,38 is electro-chemically polymerizable in an aqueous solution,39,40 and has been shown to exhibit significantly better electrical conductivity and chemical stability than polypyrrole.41 Thus a PEDOT component was used in place of the polypyrrole component to enhance the stability of the NIDE glucose biosensor. Figure 1 (right) shows a topographic image of the morphology of the p(HEMA)-PEDOTPEGMA composite hydrogel after direct electrochemical deposition at +0.850 volt for 100 seconds of the PEDOT component into the p(HEMA)-PEGMA hydrogel on the electroactive window of the NIDE biosensor. The film turned translucent blue–black in appearance. The surface morphology is nodular and highly porous. Consequently, a large amount of electrolyte solution can gain access through the composite hydrogel to the NIDE. This porous structure allows fast transport of ions in the supporting electrolyte solution; as a result, the effective diffusion length of the ions in the conducting polymer is much shorter, thus resulting in a fast steady-state response time.
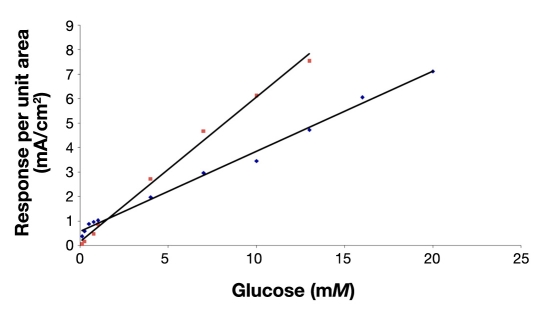
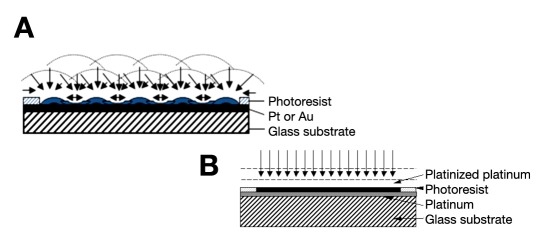
Figure 2 shows the corresponding amperometric response curve of the composite hydrogel NIDE glucose biosensor and previous composite hydrogel planar (nonindented) glucose biosensor in phosphate-buffered saline (7.4) to various concentrations of glucose. At 25°C, the dynamic linear range for the planar glucose biosensor was 0.10–13.0 mM (biosensor response (μA) = 0.5906 [glucose] (mM) + 0.1624 (μA); r2 = 0.994) and 0.25–20.0 mM for the NIDE glucose biosensor. The linear equation for the NIDE glucose biosensor was biosensor response (μA) = 0.323 [glucose] (mM) + 0.634 (μA) (r2 = 0.994). The time taken to reach 95% of the steady-state response was 5 seconds compared to that of 50 seconds for the planar glucose biosensor. The NIDE glucose biosensor demonstrated a swift response time for the composite hydrogel NIDE biosensors employing PEDOT, PEGMA, and cross-linked p(HEMA). Thus, by changing the electrode design from a planar to a NIDE configuration and incorporating a PEDOT component in the hydrogel formulation, we incurred a significant reduction of the flux of enzymatically generated H2O2 to the underlying exposed platinized regions of the polarized gold NIDE. Moreover, we simultaneously promoted spherical/radial diffusion to the nanoindents of the NIDEs. This has the effect of extending the linear response range of the NIDE glucose biosensor by approximately two times that achieved by the planar glucose biosensor.4 Figure 3 schematically represents the effect of electrode geometry on the mass-transport diffusional characteristics of the substrate at the active electrode area. The platinized sites of the NIDEs (Figure 3A) provide microscopic surface topographies that promote radial mass transport of the substrate at the electrode surface. The platinized planar electrode geometry (Figure 3B) provides the equivalent electrode area that, in effect, causes the corresponding radial diffusion layers to overlap with one another and so produces the familiar, limiting situation of semi-infinite linear diffusion.42,43
Figure 2.
Amperometric response curve of glucose biosensors having a platinized NIDE design (blue ♦) and planar design (pink ▪). Conditions were 0.01 M KH2PO4, pH 7.4, and 25°C.
Figure 3.
Schematic representation of the conversion of radial to linear diffusion of the substrate at (A) the platinized NIDE design and hemispherical diffusion and (B) platinized planar electrode geometry. Pt, platinum; Au, gold.
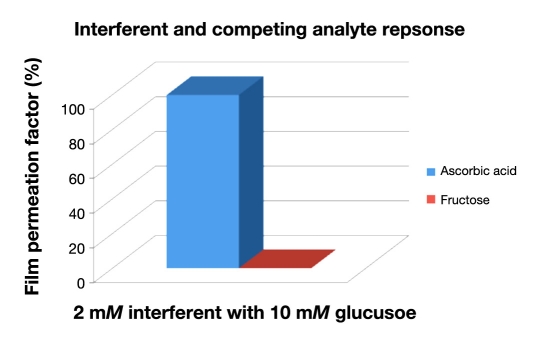
The ability of the polymeric [p(HEMA-PEDOT-PEGMA)] membrane of the NIDE glucose biosensor to effectively screen interferents from reaching the electrode surface was quantified by a parameter called the membrane permeation factor, which is defined as the amperometric response of the polymer-coated electrode to the interferent divided by the amperometric response of the bare electrode to the interferent multiplied by 100. This parameter was determined for a mixture containing two endogenous interferents—ascorbic acid and fructose— at the p(HEMA-PEDOT-PEGMA) membrane. The effect of the electroactive and competing analyte to the glucose response of p(HEMA-PEDOT-PEGMA) was examined in the presence of 2 mM of the respective interferents with a 10 mM glucose concentration. Results are depicted in Figure 4. It can be observed that ascorbic acid was highly permeable to the p(HEMA-PEDOT-PEGMA) membrane, whereas fructose was nearly impermeable to the composite membrane and does not have a significant effect on the glucose biosensor. As such, the proposed glucose biosensor demonstrates increased access to ascorbic acid.
Figure 4.
The effect of 2 mM electroactive interferents and competing analyte to the glucose response of p(HEMA-PEDOT-PEGMA) biosensors was examined with a glucose concentration of 10 mM in the presence of the NIDE glucose biosensor.
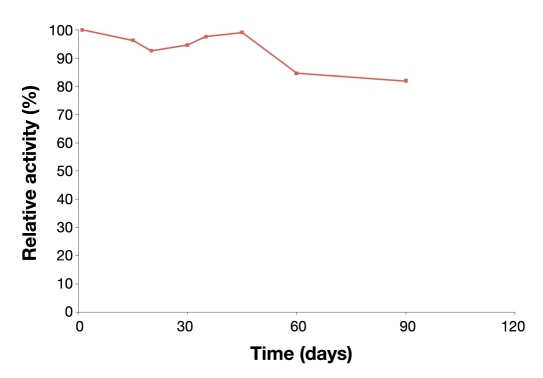
The NIDE design is superior to the planar and other conventional electrode designs because the nanoindents result in a semisphere-like pattern with many more active sites than simple planar transducers, which leads to the promotion of hemispherical diffusion, while providing an increase in the number of active sites for high enzyme loading, retention of bioactivity (hence stability), and robust detection, thus enabling them to be more effective biosensor systems. Another benefit of the nanopattern biosensor is that it is a more efficient trap for the enzyme and conversion of H2O 2to electrons in the “cavities” of the NIDE surfaces so that much more current is generated in comparison to flat planar structures. Application of nanoscale effects of a transducer permits the confinement of enzymes in the “cavities,” which increased the stability of the enzymes considerably. While the majority of electrochemical glucose biosensors reported in the literature may have linear dynamic ranges that encompass the basal concentration range, far fewer have been reported with an upper limit approaching the 20 mM that has been achieved with the present NIDE biosensor. This suggests that enzymes immobilized within the PEDOT and PEGMA composite hydrogel do not compromise this analytical parameter of the biosensor. Enzyme immobilization is reflected in the stability profiles of Figure 5, which shows storage stability data for the NIDE glucose biosensor measured periodically over 3 months of storage at 4°C in the absence of buffer. These values are amperometric responses of the NIDE glucose biosensor to 10 mM glucose expressed as a percentage of relative activity of the respective initial biosensor response. The systems exhibited good profiles within the first month of storage and showed a lesser decline in activity beyond 30 days of storage. After 3 months of storage, the immobilized enzyme system retained 82% of its initial activity.
Figure 5.
Storage stability profiles for the NIDE glucose biosensor. Glucose oxidase was immobilized in cross-linked p(HEMA) hydrogel membranes containing PEDOT and stored dry at 4°C when not in use. Amperometric responses were in response to a 10 mM glucose challenge in phosphate-buffered saline, pH 7.4, at 37°C.
Conclusion
Design of a NIDE glucose biosensor that integrates soft-condensed, biosmart hydrogels and has the potential for long-term in vitro monitoring of clinically important analytes such as glucose monitoring of clinically important analytes such as glucose was described. The sensing membrane layer consists of three main components: cross-linked p(HEMA), which serves as the hydrophilic network backbone; a PEDOT network, which acts as a barrier to interferents; and the PEG chains tethered to the polymer backbone to stabilize enzyme activity. The NIDE glucose biosensor resulted in generation of a higher peak current and increased dynamic range and overcame the limited number of active site problems associated with most conventional electrodes. These results suggest that the NIDE contributed to the increase in the dynamic range, improvement of the device sensitivity, and response time. When applied to a glucose biosensor, the NIDE design demonstrated linearity up to 20 mM glucose, approximately twofold greater than the linear dynamic range for a corresponding planar glucose biosensor. Moreover, successful enhancement of the selectivity of the composite hydrogel membrane layers utilized in fabrication of the NIDE biosensor depends on the proper characterization of ion and small molecule transport through various composite hydrogel formulations. Work is currently underway on various composite membranes to effectively screen out interferents, such as ascorbic acid. The nanoindentation method described here can be adapted easily to other types of enzyme-based biosensors, and this work is in progress in the laboratory.
Acknowledgment
The author thanks the Virginia State University Center for Biosystems and Engineering.
Abbreviations
- AFM
atomic force microscopy
- GOx
enzyme glucose oxidase
- HEMA
hydroxyethyl methacrylate
- NIDE
nanoindented electrode
- PEDOT
poly(3,4-ethylenedioxythiophene)
- PEG
poly(ethylene glycol)
- PEGMA
poly(ethylene glycol methacrylate)
- PME
planar microelectrode
- TEGDA
tetraethyleneglycol diacrylate
- UV
ultraviolet
References
- 1.Wolfbeis OS, Oehme I, Papkovskaya N, Klimant I. Sol-gel based glucose biosensors employing optical oxygen transducers, and a method for compensating for variable oxygen background. Biosens Bioelectron. 2000;15(1–2):69–76. doi: 10.1016/s0956-5663(99)00073-1. [DOI] [PubMed] [Google Scholar]
- 2.Pigman W, Horton D. Oha carbohydrates: chemistry and biochemistry. Vols. IA, IB, IIA, IIIB. New York: Academic; 1970, 1972, 1980. [Google Scholar]
- 3.Lehninger AL, Nelson DL, Cox MM. 2nd ed. New York: Worth; 1993. Principles of biochemistry. [Google Scholar]
- 4.Guiseppi-Elie A, Brahim S, Slaughter G, Ward KR. Design of a subcutaneous implantable biochip for monitoring of glucose and lactate. IEEE Sens J. 2005;5(3):345–355. [Google Scholar]
- 5.Clark LC, Jr, Lyons C. Electrode systems for continuous monitoring in cardiovascular surgery. Ann N Y Acad Sci. 1962;102:29–45. doi: 10.1111/j.1749-6632.1962.tb13623.x. [DOI] [PubMed] [Google Scholar]
- 6.Updike SJ, Hicks GP. The enzyme electrode. Nature. 1967;214(92):986–988. doi: 10.1038/214986a0. [DOI] [PubMed] [Google Scholar]
- 7.Cosnier S. Biomolecule immobilization on electrode surfaces by entrapment or attachment to electrochemically polymerized films. A review. Biosens Bioelectron. 1999;14(5):443–456. doi: 10.1016/s0956-5663(99)00024-x. [DOI] [PubMed] [Google Scholar]
- 8.Tinkilic N, Cubuk O, Isildak I. Glucose and urea biosensors based on all solid-state PVC-NH2 membrane electrodes. Anal Chim Acta. 2002;452:29–34. [Google Scholar]
- 9.Marquette CA, Blum LJ. Luminol electrochemiluminescence-based fibre optic biosensors for flow injection analysis of glucose and lactate in natural samples. Anal Chim Acta. 1999;381(1):1–10. [Google Scholar]
- 10.Raba J, Mottola HA. Glucose oxidase as an analytic reagent. Crit Rev Anal Chem. 1995;25(1):1–42. [Google Scholar]
- 11.De Marcos S, Galindo J, Sierra JF, Galbán J, Castillo JR. An optical glucose biosensor based on derived glucose oxidase immobilised onto a sol-gel matrix. Sens Actuators B. 1999;57(1–3):227–232. [Google Scholar]
- 12.Gunasingham H, Tan CH, Aw TC. Comparative study of first-, second- and third-generation amperometric glucose enzyme electrodes in continuous-flow analysis of undiluted whole blood. Anal Chim Acta. 1990;234:321–330. [Google Scholar]
- 13.Turner A. Biosensors. Curr Opin Biotechnol. 1994;5(1):49–53. doi: 10.1016/s0958-1669(05)80069-2. [DOI] [PubMed] [Google Scholar]
- 14.Wisniewski N, Reichert M. Methods for reducing biosensor membrane biofouling. Colloids Surf B Biointerfaces. 2000;18(3–4):197–219. doi: 10.1016/s0927-7765(99)00148-4. [DOI] [PubMed] [Google Scholar]
- 15.Cosnier S, Szunerits S, Marks RS, Novoa A, Puech L, Perez E, Rico-Lattes I. A comparative physical study of two different hydrophilic synthetic latex matrices for the construction of a glucose biosensor. Talanta. 2001;55(5):889–897. doi: 10.1016/s0039-9140(01)00498-2. [DOI] [PubMed] [Google Scholar]
- 16.Revzin AF, Sirkar K, Simonian A, Pishko MV. Glucose, lactate, and pyruvate biosensor arrays based on redox polymer/oxidoreductase nanocomposite thin-films deposited on photolithographically patterned gold microelectrodes. Sens Actuators B. 2002;81(2–3):359–368. [Google Scholar]
- 17.Quinn CP, Pathak CP, Heller A, Hubbell JA. Photo-crosslinked copolymers of 2-hydroxyethyl methacrylate, poly(ethylene glycol) tetra-acrylate and ethylene dimethacrylate for improving biocompatibility of biosensors. Biomaterials. 1995;16(5):389–396. doi: 10.1016/0142-9612(95)98856-9. [DOI] [PubMed] [Google Scholar]
- 18.Lowy DA, Finklea HO. Gold electrodes with polyion multilayers and electrostatically bound redox couples. Electrochim Acta. 1997;42(9):1325–1335. [Google Scholar]
- 19.Ward WK, House JL, Birck J, Anderson EM, Jansen LB. A wire-based dual-analyte sensor for glucose and lactate: in vitro and in vivo evaluation. Diabetes Technol Ther. 2004;6(3):389–401. doi: 10.1089/152091504774198106. [DOI] [PubMed] [Google Scholar]
- 20.Yu B, Ju Y, West L, Moussy Y, Moussy F. An investigation of long-term performance of minimally invasive glucose biosensors. Diabetes Technol Ther. 2007;9(3):265–275. doi: 10.1089/dia.2006.0020. [DOI] [PubMed] [Google Scholar]
- 21.Schuvailo OM, Soldatkin OO, Lefebvre A, Cespuglio R, Soldatkin AP. Highly selective microbiosensors for in vivo measurement of glucose, lactate and glutamate. Anal Chim Acta. 2006;110(6):573–574. doi: 10.1016/j.aca.2006.03.034. [DOI] [PubMed] [Google Scholar]
- 22.Gough DA, Armour JC, Baker DA. Advances and prospects in glucose assay technology. Diabetologia. 1997;40(Suppl 2):102–107. doi: 10.1007/s001250051418. [DOI] [PubMed] [Google Scholar]
- 23.Chen T, Qian YZ, Di X, Rice A, Zhu JP, Bullock R. Lactate/glucose dynamics after rat fluid percussion brain injury. J Neurotrauma. 2000;17(2):135–142. doi: 10.1089/neu.2000.17.135. [DOI] [PubMed] [Google Scholar]
- 24.Davies TJ, Compton RG. The cyclic and linear sweep voltammetry of regular and random arrays of microdisc electrodes. Theory. 2005;585(1):63–82. [Google Scholar]
- 25.Morita M, Niwa O, Horiuchi T. Interdigitated array microelectrodes as electrochemical sensors. Electrochim Acta. 1997;42(20–22):3177–3183. [Google Scholar]
- 26.González-García O, Ariño C, Esteban M. Chronoamperometric and voltammetric characterization of gold ultramicroelectrode arrays. Electroanalysis. 2007;19(4):429–435. [Google Scholar]
- 27.Brahim S, Narinesingh D, Guiseppi-Elie A. Polypyrrole-hydrogel composites for the construction of clinically important biosensors. Biosens Bioelectron. 2002;17(1–2):53–59. doi: 10.1016/s0956-5663(01)00262-7. [DOI] [PubMed] [Google Scholar]
- 28.Guiseppi-Elie A, Brahim S, Narinesingh D. Composite hydrogels containing polypyrrole as support membranes for amperometric enzyme biosensors. J Macromol Sci A. 2001;38(12):1575–1591. [Google Scholar]
- 29.Magno R, Bennett BR. Nanostructure patterns written in III-V semiconductors by an atomic force microscope. Appl Phys Lett. 1997;70(14):1855–1857. [Google Scholar]
- 30.Lee D, Wei X, Chen X, Zhao M, Jun S, Hone J, Herbert E, Oliver W, Kysar J. Microfabrication and mechanical properties of nanoporous gold at the nanoscale. Scripta Materialia. 2007;56(5):437–440. [Google Scholar]
- 31.Schwartz PV. Meniscus force nanografting: nanoscopic patterning of DNA. Langmuir. 2001;17(19):5971–5977. [Google Scholar]
- 32.Joo H, Yoo YJ, Ryu DD. A biosensor stabilized by polyethylene glycol for the monitoring of hydrogen peroxide in organic solvent media. Enzyme Microbial Technol. 1996;19(1):50–56. [Google Scholar]
- 33.Harris JM. In: Introduction to biotechnical and biomedical applications of poly(ethylene glycol) Harris JM, editor. New York: Plenum Press; 1992. pp. 1–14. Poly(ethylene glycol) chemistry: biotechnical and biomedical applications. [Google Scholar]
- 34.Peppas NA, Keys KB, Torres-Lugo M, Lowman AM. Poly(ethylene glycol)-containing hydrogels in drug delivery. J Control Release. 1999;62(1–2):81–87. doi: 10.1016/s0168-3659(99)00027-9. [DOI] [PubMed] [Google Scholar]
- 35.Kros A, van Hövell SW, Sommerdijk NA, Nolte RJ. Poly(3,4 ethylenedioxythiophene)-based glucose biosensors. Adv Mater. 2001;13(20):1555–1557. [Google Scholar]
- 36.Smela E. Conjugated polymer actuators for biomedical applications. Adv Mater. 2003;15(6):481–494. [Google Scholar]
- 37.Jonas F, Schrader L. Conductive modifications of polymers with polypyrroles and polythiophenes. Synth Metals. 1991;41–43:831–836. [Google Scholar]
- 38.Dietrich M, Heinze J, Heywang G, Jonas F. Electrochemical and spectroscopic characterization of polyalkylenedioxythiophenes. J Electroanal Chem. 1994;369:87–92. [Google Scholar]
- 39.Pei Q, Zuccarello G, Ahlskog M, Inganäs O. Electrochromic and highly stable poly(3,4-ethylenedioxythiophene) switches between opaque blue-black and transparent sky blue. Polymer. 1994;35(7):1347–1351. [Google Scholar]
- 40.Carlberg JC, Inganäs O. Poly(3,4-ethylenedioxythiophene) as electrode material in electrochemical capacitors. J Electrochem Soc. 1997;144(4):L61–L64. [Google Scholar]
- 41.Yamato H, Ohwa M, Wernet W. Stability of polypyrrole and poly(3,4-ethylenedioxythiophene) for biosensor application. J Electroanal Chem. 1995;397(1–2):163–170. [Google Scholar]
- 42.Armstrong FA, Bond AM, Hill AO, Oliver BN, Psalti IS. Electrochemistry of cytochrome c, plastocyanin, and ferredoxin at edge- and basal-plane graphite electrodes interpreted via a model based on electron transfer at electroactive sites of microscopic dimensions in size. J Am Chem Soc. 1989;111(26):9185–9189. [Google Scholar]
- 43.Bard AJ, Faulkner LR. Electrochemical methods: fundamentals and applications. New York: John Wiley and Sons; 1980. [Google Scholar]