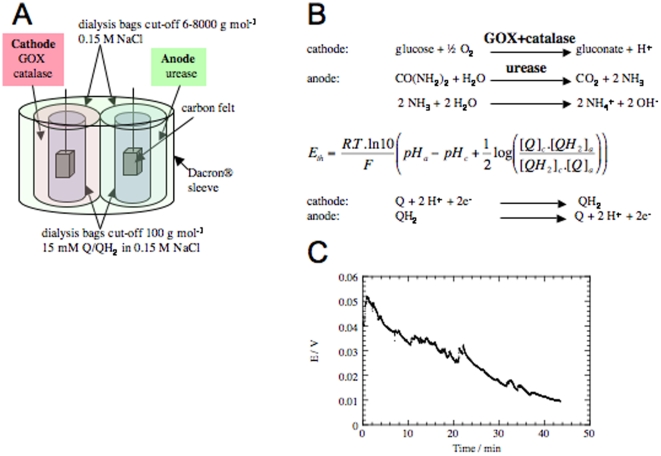
Figure 5. Implantable “Quinhydrone pH-based Glucose and Urea BioFuel Cell” with mechanically confined electrodes.
(A) Schematic representation. In each electrode, the redox species, quinone (Q) and hydroquinone (QH2) are confined close to a carbon felt by a first dialysis bag (nominal cut-off of 100 g mol−1). This bag separates the redox species from the enzymes contained in a second dialysis bag with a nominal cut-off of 6–8000 g mol−1. This second dialysis bag contains GOX and catalase for the anode, and urease for the cathode. The two electrodes are packed together in a Dacron® sleeve. (B) Electro-chemical reactions at the electrodes. Action of the GOX at the cathode locally decreases the pH, while action of the urease at the anode locally increases pH. Nernst law governs the difference of potential between anode and cathode (subscripts a is used in this equation to identify pH and concentrations of species at the anode, subscript c denoting the cathode). At the cathode, quinone (Q) is reduced into hydroquinone (QH2), while at the anode hydroquinone (QH2) is oxidised into quinone (Q). (C) Discharge curve under 100 nA. This curve was recorded after implantation in the retroperitoneal space of a rat, for a constant current of 100 nA. It corresponds to a mean power of 3 nW during 45 minutes.