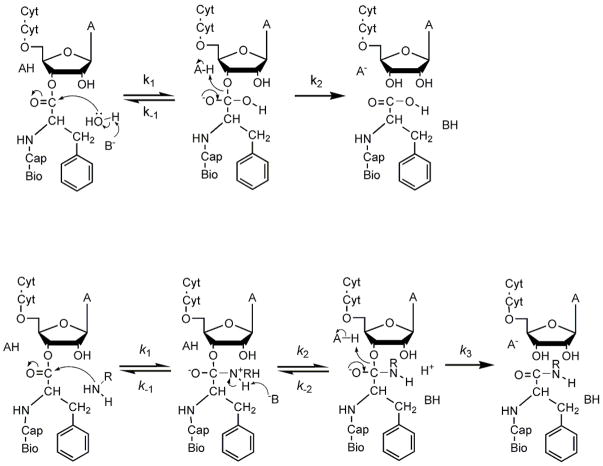
Figure 3.
Possible reaction mechanisms of CCApcb. A) Hydrolysis. Attack by hydroxide ion or base-catalyzed attack of water results in the formation of a tetrahedral intermediate. Breaking the ester C-O bond resolves the intermediate into products. In some cases the intermediate resolves back into reactants. B) Aminolysis. Amines can attack as neutral species, and the zwitterionic state may be a stable intermediate as shown. Alternatively, one or more steps may be concerted. Because NRH2 is generally a better leaving group than RO−, the intermediates will often partition back to substrates.