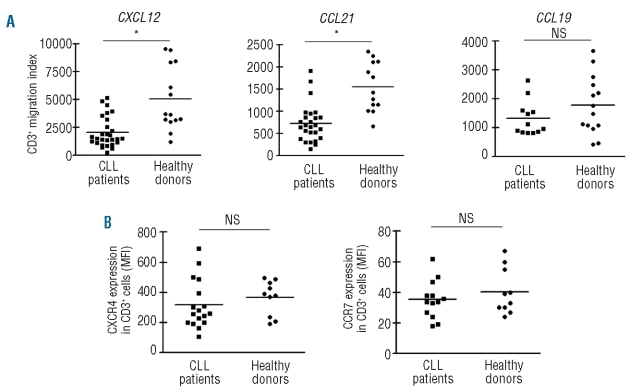
Figure 1.
T-cell migration towards CXCL12, CCL21 and CCL19 and the expression of CXCR4 and CCR7 in T cells from healthy donors and CLL patients. (A) PBMC from CLL patients or healthy adults were placed in the upper chamber of a 24-transwell plate; medium alone (control wells) or with the optimal dose of CXCL12 (1 μg/mL), CCL21 (1 μg/mL) or CCL19 (5 μg/mL) as chemoattractant was placed in the lower chamber. After 2 h of culture the migrating cells were suspended and divided into aliquots for counting with a FACSCalibur or for immunophenotyping. The migration index for each sample was calculated by determining the ratio of migrated T cells in chemokine-treated wells versus control wells, taking the spontaneous migration in control wells as 100%. NS: not significant; *P<0.01 Mann-Whitney test. (B) Freshly collected whole blood from CLL patients or healthy donors was incubated with saturating concentrations of PerCP-conjugated anti-CD3 antibodies and PE-conjugated anti-CXCR4 (clone 12G5) or anti-CCR7 (clone 3D12), or the appropriate isotype control antibody for 30 min at 4ºC. After selective red blood cell lysis the white cell pellet was evaluated by flow cytometry. Results are expressed as the mean fluorescence intensity (MFI) of CXCR4 and CCR7 expression on CD3+ cells for each sample analyzed. NS: not significant, Mann Whitney test.