Figure 3.
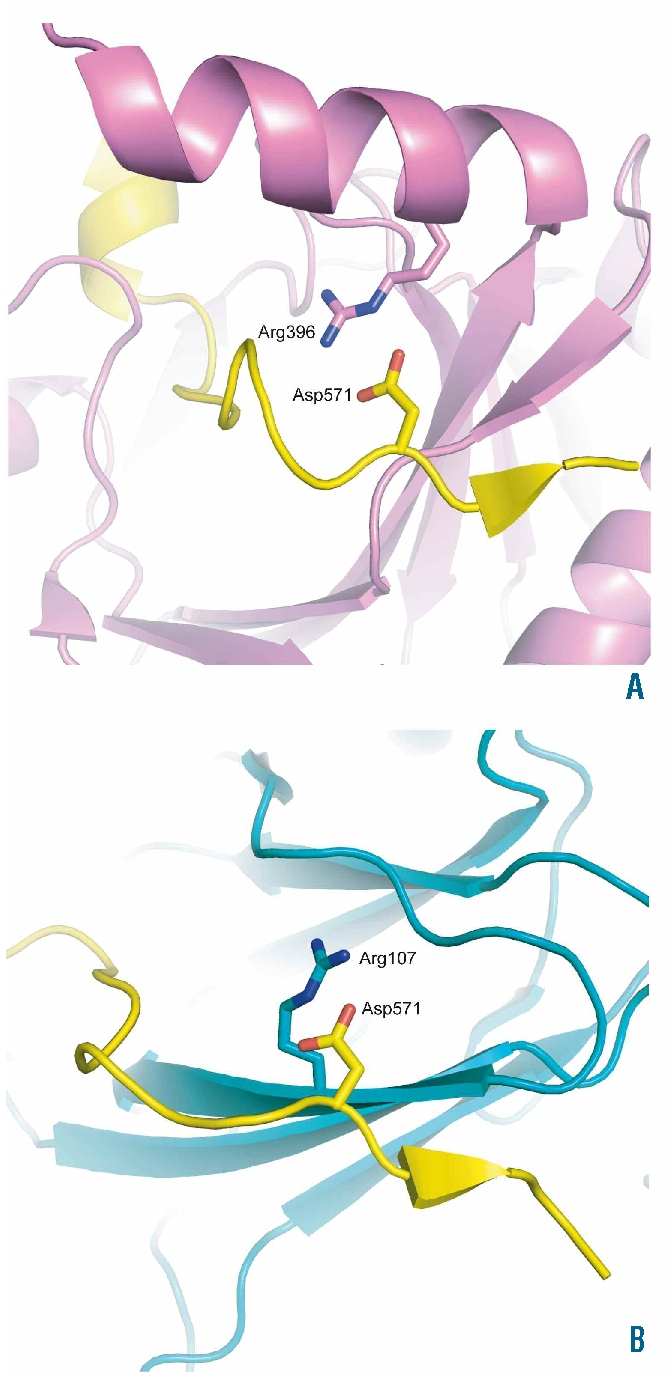
Structural effects. Images showing the position of the HIF-1α residue, Asp571, that is equivalent to HIF-2α Asp539 in the cocrystal structures of the CODD domain of HIF-1α with PHD2 (PDB ID 3HQR),21 and VHL (PDB ID 1LM8)22 (Panels A and B, respectively). HIF-1α is colored yellow, PHD2 violet, and VHL teal. HIF-1α residue Asp571 and selected residues are shown in stick representation. One of the contacts involved in HIF-1α binding to PHD2 and VHL involves the formation of salt bridges and hydrogen bonds with Arg396 of PHD2 (Panel A), and Arg107 of VHL (Panel B). In both cases, incorporation of the longer side chain of glutamic acid at position 571 in HIF-1α is likely to disrupt these interactions. The figure was generated using the program PyMOL (DeLano, W.L. The PyMOL Molecular Graphics System (2002) on World Wide Web http://www.pymol.org).
