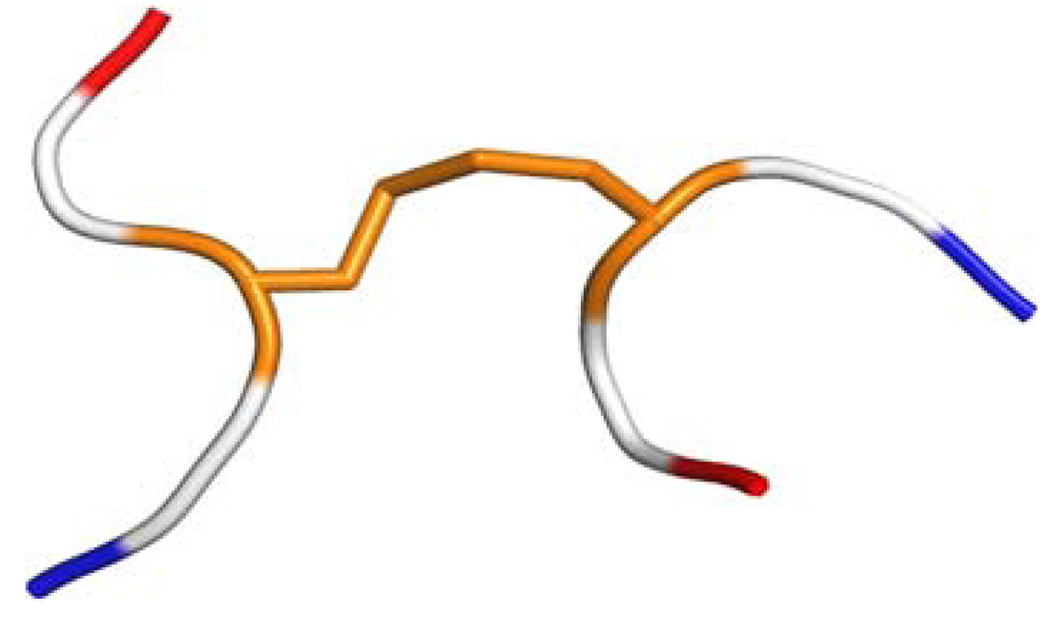
Fig. 1.
Ribbon diagram of the hexapeptide model used to compute the 13Cα and 13Cβ chemical shifts for cysteines in disulfide bonds. The hexapeptide is formed by two tripeptides Ac-GCiG-NMe and Ac-GCjG-NMe, with Ci and Cj and the disulfide bond in the conformation of the regularized experimental protein structure, and the Gly, Ac and NMe residues in their energy-minimized structure. The Cys residues with their disulfide bond are colored in orange, the Gly residues are colored white and the blocking end-groups are red and blue for Ac and NMe, respectively