Abstract
Mutations in APC/β-catenin resulting in an aberrant activation of Wnt/β-catenin pathway are common in colorectal cancer (CRC), suggesting that targeting the β-catenin pathway with chemopreventive/anticancer agents could be a potential translational approach to control CRC. Using human CRC cell lines harboring mutant (SW480) versus wildtype (HCT116) APC gene and alteration in β-catenin pathway, herein we performed both in vitro and in vivo studies to examine for the first time whether silibinin targets β-catenin pathway in its efficacy against CRC. Silibinin treatment inhibited cell growth, induced cell death, and decreased nuclear and cytoplasmic levels of β-catenin in SW480 but not in HCT116 cells, suggesting its selective effect on the β-catenin pathway and associated biologic responses. Other studies, therefore, were performed only in SW480 cells where silibinin significantly decreased β-catenin-dependent T-cell factor-4 (TCF-4) transcriptional activity and protein expression of β-catenin target genes such as c-Myc and cyclin D1. Silibinin also decreased cyclin-dependent kinase 8 (CDK8), a CRC oncoprotein that positively regulates β-catenin activity, and cyclin C expression. In a SW480 tumor xenograft study, 100- and 200-mg/kg doses of silibinin feeding for 6 weeks inhibited tumor growth by 26% to 46% (P < .001). Analyses of xenografts showed that similar to cell culture findings, silibinin decreases proliferation and expression of β-catenin, cyclin D1, c-Myc, and CDK8 but induces apoptosis in vivo. Together, these findings suggest that silibinin inhibits the growth of SW480 tumors carrying the mutant APC gene by down-regulating CDK8 and β-catenin signaling and, therefore, could be an effective agent against CRC.
Introduction
According to the American Cancer Society, 106,100 and 40,870 new cases of colon and rectal cancers would have occurred in the United States in 2009 alone, respectively. Although the incidence of colorectal cancer (CRC) has decreased during the last two decades, still it accounts for 9% of total deaths caused by cancer of all other sites [1]. Screening of adults older than 50 years has contributed significantly in reducing CRC incidence [2–4]; however, significant mortality and morbidity are still associated with clinically advanced CRC cases. Similarly, there is sufficient evidence that use of nonsteroidal anti-inflammatory drugs including aspirin and other cyclooxygenase-2 inhibitors is effective in reducing the risk of CRC [5]; however, the success of such strategies is limited by associated toxicities and adverse effects including gastrointestinal bleeding, kidney failure, etc. [5]. These limitations argue the need for the identification of mechanism-based new agents that could be useful for effective chemopreventive and/or interventional strategies against CRC.
Because genetic predispositions and lifestyle are two major risk factors for CRC [6,7], the latter one provides an opportunity to control the risk by changing daily habits. In lifestyle risk factors, dietary habits have considerable role where diets rich in fat (such as red meat) and high calories are associated with a high risk of developing CRC and those rich in fruits and vegetables have been associated with lower risk [6]. Although no conclusive studies are available in literature in this regard [8–10], fruits and vegetables not only are considered a good source of fiber, vitamins, and other nutrients, which may contribute in preventing the occurrence of CRC, but also contain nonnutrient phytochemicals with significant chemopreventive potential. To date, many food/plant-derived chemopreventive agents that exhibit strong efficacy against various cancers in in vitro and preclinical models have been identified [11–14]. We have shown that silibinin, an active ingredient of the commonly used health supplement milk thistle (Silybum marianum) extract, exhibits anticancer/chemopreventive efficacy in different in vivo and in vitro models of various cancers, including CRC [15–22]. Importantly, silibinin is largely nontoxic and is well tolerated without causing any adverse effects when given up to a dose of 1% in diet or 750 mg/kg body weight in our previously completed studies in rodents [15–22].
Aberrant Wnt signaling has been reported to contribute to various human diseases including CRC [23]. Wnt signaling pathway plays a central role in numerous cellular processes starting from embryonic development to tissue/organ homeostasis in adults. β-Catenin is a key component of this pathway, performing a dual function, being a component of cell-cell adhesion and a transcriptional activator in conjunction with T-cell factor/lymphoid enhancer factor (TCF/LEF) transcription factors [24]. The cellular levels of β-catenin are tightly regulated by a multiprotein destruction complex consisting of adenomatous polyposis coli (APC), axin, and glycogen synthase kinase 3β (GSK-3β) [25]. Aberrant activation of β-catenin, mostly due to mutation(s) in APC, confers oncogenic potential by activating several target genes of the Wnt/β-catenin pathway such as cyclin D1, c-Myc, etc. [26]. Germ line mutations in the APC gene are seen in patients whose conditions have been diagnosed as familial adenomatous polyposis or CRC [27]. In addition, activating mutations in β-catenin involving exon 3 occur in less than 5% of CRC [28,29]. Overall, an aberrant activation of β-catenin-dependent signaling has a major contribution in the pathogenesis of CRC, and therefore, targeting this pathway might have vital implications in controlling the progression of this malignancy. Herein, we studied the possible efficacy and associated mechanisms of silibinin against the human colorectal carcinoma SW480 cell line that harbors mutation in the APC gene [30] as well as in theHCT116 cell line. For the first time, we show that, in comparison to HCT116 cells, silibinin selectively inhibits the growth of SW480 cells in vitro as well as in vivo through the down-regulation of β-catenin signaling.
Materials and Methods
Reagents
TCF-luciferase reporter plasmids (8x TOP/FOP FLASH) were generously provided by Randall Moon (University of Washington, School of Medicine, Seattle, WA). Antibodies against cyclin-dependent kinase 8 (CDK8), cyclins C and D1, β-catenin, and c-Myc were from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse monoclonal antiproliferating cell nuclear antigen (PCNA) antibody was from Dako (Carpinteria, CA). Silibinin and anti-β-actin antibody were from Sigma- Aldrich (St Louis, MO). Antibody against glutathione-S-transferase pi (GST-π) was from Medical and Biological Laboratories Co, Ltd (Woburn, MA). Anti-histone H1 antibody was from Neomarkers (Fremont, CA). Alexa Fluor 488-tagged goat antirabbit antibody was from Invitrogen Corporation (Carlsbad, CA).
Cell Culture and Cell Viability Assay
SW480 and HCT116 cells were procured from American Type Culture Collection (Manassas, VA) and maintained in Leibovitz medium and Dulbecco's modified Eagle medium, respectively, supplemented with 10% FBS and 100 U/ml of penicillin and streptomycin under standard culture conditions. Cells were cultured at a density of 5000 cells/cm2 in 60-mm dishes and were treated the following day with either DMSO alone or silibinin (50-200 εM) in DMSO for 24 to 72 hours, and both adherent and nonadherent cells were collected by brief trypsinization followed by centrifugation. Cells were stained with Trypan blue and counted as live (unstained) and dead (blue-colored) cells using hemocytometer under a light microscope.
Western Immunoblot Analysis
At the end of treatments, total cell lysates were prepared in nondenaturing lysis buffer as described previously [31]. In some experiments, after treatments, nuclear and cytoplasmic fractions were prepared as described earlier [31]. Total cell lysates (50–70 εg) and nuclear or cytoplasmic fraction (5–15 εg) proteins were denatured in 2x sample buffer and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis on 8%, 12%, or 16% Tris-glycine gel [16,19]. To ensure equal protein loading, each membrane was stripped and reprobed with anti-β-actin (total cell lysates), anti-GST-π (cytosolic), or histone H1 (nuclear) antibody to normalize for differences in protein loading and to establish the purity of the cytoplasmic and nuclear fractions.
Immunofluorescence Confocal Microscopy
SW480 cells were plated on coverslips overnight and then treated with either DMSO or silibinin (100 εM) in DMSO for 48 hours. At the end of treatment time, cells grown on the coverslips were washed gently with PBS and fixed in 3% formaldehyde for 10 minutes. Cells on the coverslips were again washed twice with PBS, incubated with Triton X-100 for 5 minutes, and later washed twice with PBS followed by a final wash with TBST (10 mM Tris-HCl, pH 8.0, 150 mM NaCl, 0.2% vol/vol Tween-20). Cells were incubated in Leibovitz complete medium for 3 to 4 hours followed by β-catenin antibody for overnight. Finally, cells were incubated (45 minutes) with Alexa Fluor 488-tagged goat antirabbit secondary antibody and counterstained with 4′,6′-diamidino-2-phenylindole (DAPI) for 5 minutes. Cell images were captured at 1000x magnification on a Nikon D Eclipse C1 confocal microscope (Nikon, Instruments Inc., Melville, NY), and images were processed by EZ-C1 Freeviewer software.
TOP/FOP FLASH Activity
SW480 cells were plated at a confluence of 40% to 50% in 60-mm dishes overnight. The cells were transfected with 1 εg of 8x TOP/FOP FLASH plasmid DNA along with 0.3 εg of pRL-CMV plasmid using Mirus transfection reagent. After 24 hours of transfection, cells were treated with either DMSO or silibinin (100 εM) in DMSO for another 24 hours, and luciferase activity was measured using the Dual Luciferase Reporter Assay System (Promega, Madison, WI). Transfection efficiency was normalized using renilla luciferase activity. Experiments were done at least three times, and the data are shown from a representative experiment.
Experimental Design for Tumor Xenograft Study
Six-week-old athymic (nu/nu) male nude mice were obtained from the National Cancer Institute (Bethesda, MD), housed under standard laboratory conditions, and fed autoclaved AIN-76A rodent diet (Dyets, Inc, Bethlehem, PA) and water ad libitum. The protocol for conducting this study was approved by the Institutional Animal Care and Use Committee of the University of Colorado Denver. Mice were subcutaneously injected with exponentially growing 5 x 106 SW480 cells mixed with Matrigel (1:1) in the right flank to initiate tumor growth and then divided into three groups, each having eight mice. After 24 hours, mice in control (first) group were gavaged with vehicle (0.2 ml of 0.5% [wt/vol] carboxymethyl cellulose [CMC] in saline per day), whereas animals in the second and third groups were gavaged with 100 and 200 mg/kg body weight doses of silibinin in vehicle, respectively, 5 days/week for 6 weeks. Body weight and diet consumption were recorded twice weekly throughout the study. After tumors started growing, their sizes were measured twice weekly using digital vernier caliper. Tumor volume was calculated by using the formula 0.5236L1 (L2)2, where L1 is long axis and L2 is the short axis of the tumor. At the end of the experiment, mice were killed, and tumors were excised, weighed, and fixed in buffered formalin for further analysis.
Immunohistochemical Staining for PCNA, Cyclin D1, c-Myc, CDK8, and β-Catenin
Tumor tissues fixed in 10%phosphate-buffered formalin for 12 hours were routinely processed for paraffin-embedded 4-εm sections and immunohistochemical (IHC) staining. Briefly, sections were subjected to antigen-retrieval, quenched of endogenous peroxidase activity [15], and then incubated with anti-PCNA (1:400 dilutions), anti-cyclin D1 (1:200 dilutions), anti-c-Myc (1:100 dilutions), anti-CDK8 (1:100 dilutions), or anti-β-catenin (1:100 dilutions) antibodies in a humidity chamber. Few sections were incubated with N-Universal Negative Control antibody under identical conditions. The sections were then incubated with an appropriate biotinylated secondary antibody followed by HRP-conjugated streptavidin and 3,3′-diaminobenzidine and counterstained with Harris hematoxylin. Quantification was done by counting brown colored positive cells and total number of cells at five arbitrarily selected fields in each sample at 400x magnification.
Terminal Deoxynucleotidyl Transferase-Mediating dUTP Nick End Labeling Staining for Apoptotic Cells
Apoptotic cells were detected by terminal deoxynucleotidyl transferase-mediating dUTP nick end labeling (TUNEL) staining using Dead End Colorimetric TUNEL system (Promega) following the vendor's protocol. The apoptosis was evaluated by counting TUNEL-positive cells (brown-stained) as well as the total number of cells in five randomly selected fields in each sample at 400x magnification.
Statistical and IHC Analyses
All statistical analyses were carried out with Sigma Stat software version 3.5 (Jandel Scientific, San Rafael, CA). Statistical significance of difference between the control and treated groups was determined either by Student's t-test or one-way analysis of variance followed by Bonferroni t-test. P < .05 was considered statistically significant. IHC analyses were done with a Zeiss Axioskop 2 microscope (Carl Zeiss, Inc, Jena, Germany). Microscopic images were taken by AxioCam MrC5 camera at 400x magnification and processed by AxioVision software documentation system (Carl Zeiss, Inc). Western blots were scanned with Adobe Photoshop 6.0 (Adobe Systems, Inc, San Jose, CA), and densitometric analysis was then done using Scion Image Program (National Institutes of Health, Bethesda, MD). The densitometry data are shown below each band as fold change with respective control.
Results
Silibinin Inhibits the Growth of Human CRC SW480 Cells
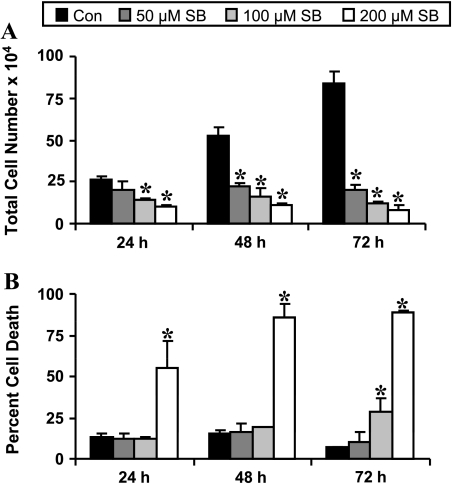
Treatment of SW480 cells with silibinin (50, 100, and 200 εM for 24–72 hours) showed a concentration- and time-dependent decrease in cell growth, where total cell number decreased by 24% to 63% (P < .001) after 24 hours, by 58% to 80% (P < .001) after 48 hours, and by 76% to 90% (P < .001) after 72 hours of 50 to 200 εM silibinin treatment, respectively (Figure 1A). Importantly, a considerable cell death was observed only at the highest concentration (200 εM) of silibinin accounting for 55% to 85% (P < .001) cell death after 24 to 48 hours of treatment (Figure 1B). However, after 72 hours of treatment, an increase in cell death was also observed at 100- (29% cell death) and 200-εM (89% cell death) concentrations of silibinin (Figure 1B). The cell death by silibinin at higher concentration/s and/or longer treatment time was apoptotic in nature (data not shown). To further examine the molecular mechanism of the growth-inhibitory effect of silibinin, we used only 50- and 100-εM silibinin concentrations, which were not cytotoxic.
Figure 1.
Silibinin inhibits the growth of human CRC SW480 cells. Cells were plated overnight and treated with 50 to 200 εM silibinin for 24 to 72 hours. At the end of treatment, cells were collected and counted on a hemocytometer after staining with Trypan blue dye under the microscope for (A) total cell number (live and dead cells together) and (B) dead cells represented as percent dead cells. Data shown are mean ±SD. *P <.001 compared with the control. SB indicates silibinin.
Silibinin Decreases Total β-Catenin Levels and Inhibits Nuclear Translocation of β-Catenin
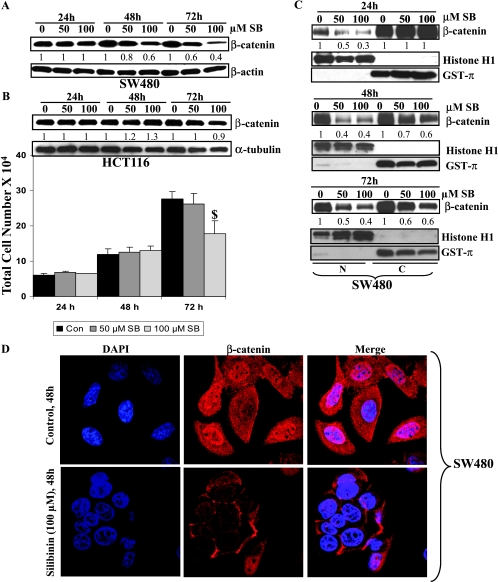
Because we observed that silibinin exerts a strong inhibitory effect on the growth of SW480 cells, we next studied whether it is mediated through modulation of the β-catenin pathway, which is aberrantly activated in this cell line. Silibinin treatment decreased total cellular pool of β-catenin in SW480 cells with a more prominent effect at 48 and 72 hours than at 24 hours (Figure 2A). To further confirm that the growth-inhibitory effect of silibinin is specifically through down-regulation of β-catenin levels and associated downstream signaling, we extended our study to another human CRC cell line, HCT116, which harbors wild-type APC but mutant β-catenin. We found that treatment of HCT116 cells with 50 and 100 εM silibinin (24–72 h) does not produce any measurable growth-inhibitory effect except at 100 εM concentration, and 72 hours of treatment time showing ∼38% decrease (Figure 2B) is contrary to its strong growth-inhibitory effect seen in SW480 cells (Figure 1A). Furthermore and more importantly, Western blot analysis revealed that silibinin treatment does not reduce β-catenin level in HCT116 cells (Figure 2B) as opposed to its strong effect in SW480 cells (Figure 2A). These observations clearly suggested an association between the growth-inhibitory activity of silibinin and a decrease in β-catenin level selectively in SW480 cells, and accordingly, we focused our efforts on this aspect only in SW480 cells.
Figure 2.
Silibinin selectively decreases the total, cytoplasmic, and nuclear pools of β-catenin in human CRC SW480 cells. Cells were plated overnight and treated with 50 to 100 εM silibinin for 24 to 72 hours. At the end of treatment, SW480 (A) and HCT116 (B) cells were analyzed for total β-catenin levels by Western immunoblot analysis. HCT116 cells were also analyzed for the total cell number after these treatments (B), and the data shown are mean ± SD. $P < .05 compared with the control. (C) In separate studies, after similar treatments of SW480 cells, cytoplasmic and nuclear fractions were prepared and analyzed for β-catenin levels by Western immunoblot analysis. Membranes were reprobed with histone H1 (N, nuclear fraction) or GST-π (C, cytoplasmic fraction) as loading controls and as markers for purity of the fractions. Densitometry data shown below each band represent fold change compared with the control after normalization with respective loading controls. (D) Cellular localization of β-catenin was also observed by immunofluorescent staining followed by confocal imaging. For this, SW480 cells grown on coverslips were treated with either DMSO alone (control) or 100 εM silibinin for 48 hours, fixed, and stained for β-catenin (red) or nuclei with DAPI (blue). Original magnification, x1000.
Because nuclear translocation of β-catenin is required for its transcriptional activity, we next studied the effect of silibinin on nuclear translocation of β-catenin. In Western blot analysis, we observed a striking decrease in the nuclear level of β-catenin after silibinin treatment at both concentrations and at all three time points studied (Figure 2C), together with a decrease in its cytoplasmic level but only after 48 and 72 hours of treatment. GST-π and histone H1 levels were also analyzed to check the purity of the cytoplasmic and nuclear fractions as well as protein loading, respectively (Figure 2C), and the fold change numbers shown below the bands are after correcting for these loading controls. We further confirmed our results about the effect of silibinin in decreasing both the cytoplasmic and nuclear β-catenin levels by immunocytochemical analysis for cellular distribution of β-catenin. As shown in Figure 2D, silibinin-treated SW480 cells (48 hours) showed overall lesser immunofluorescence (red) for β-catenin (in both cytoplasm and nucleus) compared with an intense β-catenin staining observed in the cytoplasm as well as in the nucleus of control cells.
Silibinin Inhibits β-Catenin-Dependent Transcriptional Activity and Decreases the Expression of CDK8 and Downstream β-Catenin-Transcriptional Targets
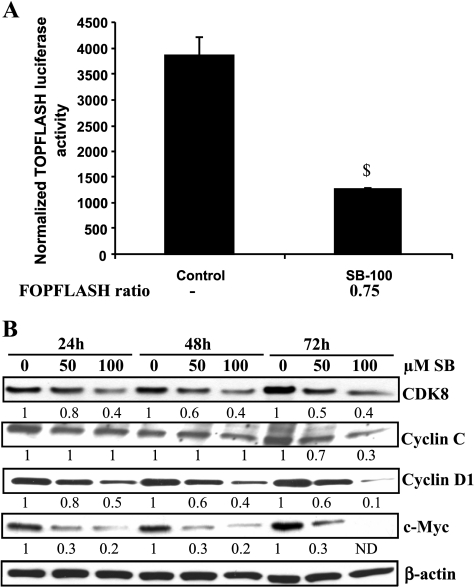
To study the effect of silibinin on the transactivation function of β-catenin, SW480 cells were transiently transfected with TOP/FOP FLASH reporter constructs that contain eight wild-type or mutant TCF/LEF binding sites upstream of the Luciferase gene. The reporter activity was significantly (P < .05) inhibited by 100 εM silibinin after 24 hours of treatment (Figure 3A), suggesting that silibinin inhibits β-catenin-mediated transcriptional activity. A marginal nonspecific inhibitory effect of silibinin (25% decrease or 0.75 FOP FLASH ratio of control and silibinin treatment) on FOP FLASH reporter activity was also observed (Figure 3A). Recently, it has been shown that transcriptional activity of β-catenin is regulated by CDK8 and cyclin C, of which CDK8 works as an oncogene in CRC [32]. We, therefore, also examined the protein levels of both CDK8 and cyclin C. Silibinin treatments reduced CDK8 level in a concentration-dependent manner at all three time points of 24 to 72 hours; however, a decrease in cyclin C level was observed only at 72 hours of treatment (Figure 3B). Thus, a decrease in the CDK8 level by silibinin might be an additional contributory mechanism in its inhibitory effect on β-catenin-mediated transcriptional activity. In other studies examining the effect of silibinin on the levels of downstream target proteins (cyclin D1 and c-Myc) of β-catenin signaling, silibinin reduced the protein levels of both cyclin D1 and c-Myc in both concentration- and time-dependent manners (Figure 3B).
Figure 3.
Silibinin inhibits β-catenin-mediated transcriptional activity and the expression of its target genes in human CRC SW480 cells. (A) Cells were plated to 40% to 50% confluence overnight and transfected with 1 εg of TOP/FOP FLASH plasmid constructs along with 300 ng of pRL-CMV plasmid for 24 hours and then treated with DMSO alone (control) or 100 εM silibinin for another 24 hours. Luciferase activity was measured using Dual Luciferase Assay kit (Promega) following the manufacturer's instruction. Data shown represent mean ± SD of three independent observations. (B) Cells were plated overnight and treated with 50–100 εM silibinin for 24 to 72 hours. Total cell lysates were then analyzed by Western immunoblot analysis for CDK8, cyclin C, cyclin D1, and c-Myc levels. Membranes were reprobed with β-actin as loading control. Densitometry data shown below each band represent fold change compared with control after normalization with respective loading controls (β-actin). $P < .05 compared with control.
Silibinin Suppresses Human CRC SW480 Tumor Xenograft Growth in Nude Mice
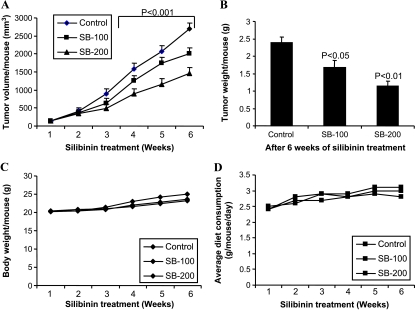
To translate our above in vitro findings in to in vivo biologic relevance, next we evaluated the effect of silibinin on SW480 tumor xenograft growth in athymic nude mice. Oral gavage feeding of silibinin at 100 and 200 mg/kg body weight doses, 5 days/week for 6 weeks, caused a marked time-dependent inhibition of xenograft growth in comparison to vehicle control (Figure 4A). At the end of the experiment, tumor volume was reduced from 2715 mm3 per mouse in the control group to 2015 and 1463 mm3 per mouse in the 100- and 200-mg/kg body weight silibinin treatment groups, which accounted for 26% and 46% decreases, respectively (P < .001; Figure 4A). Consistent with these results, silibinin treatments also showed a reduction in tumor weight by 29% and 52% (P < .05 to P < .01), respectively (Figure 4B). Silibinin feeding did not show any gross signs of toxicity or possible adverse effects assessed in body weight gain and diet consumption profiles, during 6 weeks of experiment (Figure 4, C and D). Together, these results demonstrate in vivo antitumor efficacy of oral silibinin feeding against CRC SW480 tumor xenografts without any apparent signs of toxicity.
Figure 4.
Silibinin treatment inhibits human CRC SW480 xenograft growth in athymic nude mice. Mice were subcutaneously injected with SW480 cells mixed with Matrigel and, after 24 hours, gavaged with CMC (control group) or 100 (SB-100) and 200 mg/kg body weight/day doses (SB-200) of silibinin for 5 days/week for 6 weeks. (A) Tumor volume/mouse as a function of time, (B) tumor weight/mouse at the end of study, (C) average body weight/mouse, and (D) average diet consumption/mouse/day were analyzed as detailed in Materials and Methods. Data shown in panels A and B are mean ± SE from eight mice in each group.
Silibinin Inhibits Cell Proliferation and Induces Apoptosis in Human CRC SW480 Tumor Xenografts
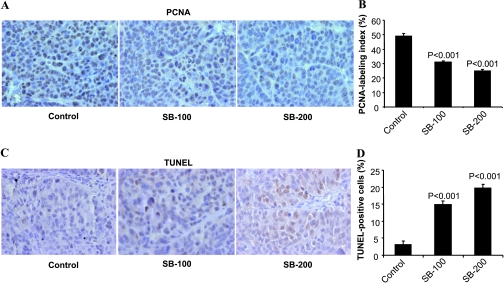
We next investigated the mechanism/s underlying the in vivo anticancer efficacy of silibinin by IHC analysis for PCNA, a marker for cell proliferation, and TUNEL, a marker for apoptotic response (Figure 5). Microscopic examination of tumor xenograft sections clearly showed a decreased immunoreactivity for PCNA in the silibinin-treated groups compared with the vehicle control (Figure 5A). Quantification of PCNA-positive cells showed 38%and 49% (P < .001) decreases in proliferation indices in silibinin-treated (100 and 200 mg/kg body weight) groups compared with vehicle control (Figure 5B). In case of apoptosis, as shown in the representative photographs, tumor xenografts from the silibinin-fed groups showed a marked increase in TUNEL-positive cells compared with the control group (Figure 5C). Quantification of TUNEL-stained samples showed five- to six-fold increases (P < .001) in the number of TUNEL-positive cells in the silibinin-treated groups compared with the control group (Figure 5D). Together, these results clearly show both antiproliferative and proapoptotic effects of silibinin in SW480 tumor xenografts as two major biologic end point mechanisms in its overall anticancer efficacy.
Figure 5.
Silibinin treatment inhibits cell proliferation and induces apoptosis in human CRC SW480 xenograft. Xenograft tumor tissues were analyzed for PCNA- and TUNEL-positive cells. Representative photographs for IHC staining of PCNA-positive (A) and TUNEL-positive (C) cells in tumor tissue from the vehicle control and silibinin-fed groups, respectively, are shown at 400x magnification. (B) Percent PCNA labeling index and (D) percent TUNEL-positive apoptotic cells in tissues were analyzed as detailed in Materials and Methods. Data shown represent mean ± SE from eight mice in each group. SB-100 and SB-200 indicates 100 and 200 mg/kg body weight silibinin, respectively.
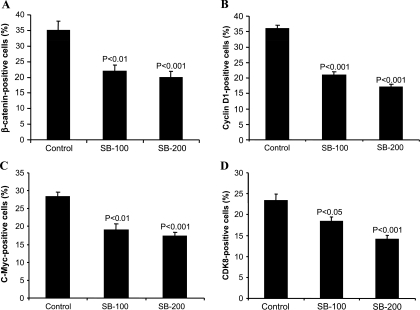
Silibinin Decreases β-Catenin, Cyclin D1, c-Myc, and CDK8 Expression in Human CRC SW480 Tumor Xenografts
Overexpression of β-catenin and its downstream target proteins is commonly observed in both experimental and human CRCs. To ascertain an in vivo effect of silibinin on the expression of β-catenin and its downstream target molecules, SW480 xenograft tissues from control and silibinin-treated groups were also analyzed by IHC staining for β-catenin, cyclin D1, and c-Myc protein levels. Silibinin-treated (100 and 200 mg/kg body weight) xenografts showed 37% and 43% (P < .01) decreases in the number of β-catenin-positive cells compared with vehicle control (Figure 6A). A similar effect of silibinin on cyclin D1 expression was also observed where it caused 39% and 52% (P < .001) decreases in cyclin D1-positive cells at two dose levels compared with control (Figure 6B). In case of c-Myc expression, the percentage of c-Myc-positive cells was reduced by 33% and 39% (P < .01 to P < .001) by these silibinin treatments (Figure 6C). Next, we studied the effect of silibinin on CDK8 expression, which regulates β-catenin-mediated transcriptional activity and seems to be one of the molecular targets of silibinin action in SW480 cells in our cell culture studies. IHC analysis of xenografts showed that silibinin treatment also significantly decreases CDK8-positive cells by 22% and 40% (P < .05 to P < .001) at two dose levels compared with vehicle control (Figure 6D). Together, these findings suggest that down-regulation of β-catenin levels and consequent signaling could be a potential in vivo mechanism by which silibinin inhibits SW480 tumor growth in nude mice.
Figure 6.
Silibinin treatment suppresses β-catenin, cyclin D1, c-Myc, and CDK8 expression in human CRC SW480 xenograft. Xenograft tumor tissues were analyzed for (A) β-catenin-, (B) cyclin D1-, (C) c-Myc-, and (D) CDK8-positive cells as detailed in Materials and Methods. Data shown represent mean ± SE from eight mice in each group.
Discussion
CRC is considered as one of the preventable malignancies because lifestyle is one of the major factors that could be changed. Although the incidence of CRC has shown decline in recent years and 5-year survival rate is 90% when diagnosed at an early and localized stage, the chances of diagnosis at this stage is only 39% [1]. Therefore, apart from screening and early diagnosis in a high-risk population, various preventive measures such as maintaining healthy lifestyle and chemoprevention strategies are expected to cause a great impact on lowering the incidence of this malignancy. Chemopreventive strategies on the basis of relatively nontoxic agents are required in light of increased cardiovascular risk and other upper gastrointestinal adverse effects with prolonged use of nonsteroidal anti-inflammatory drugs as chemopreventive agents [5]. Naturally occurring agents might serve as nontoxic alternatives to these drugs as chemopreventive agents for long-term use in high-risk targeted CRC patient population. In the present study, we studied the efficacy of silibinin, an active constituent of milk thistle extract that is traditionally used for curing hepatic ailments and has also shown chemopreventive efficacy against various epithelial malignancies in numerous in vitro and preclinical models [14–22,33,34]. In previously published studies, we demonstrated the anticancer/chemopreventive efficacy of silibinin against human colorectal carcinoma HT-29 and LoVo cells, which represent early developmental and advanced metastatic stages of CRC, respectively [15,16]. However, the effect of silibinin on the β-catenin pathway in CRC is largely unknown. Herein, we conducted a detailed mechanistic study to evaluate the efficacy of silibinin against human CRC using human CRC SW480 cells in vitro and in vivo. This cell line represents the advanced stage (Duke type B) of CRC and harbors mutant APC and wild-type β-catenin. Because APC is most frequently mutated in most cases of CRC followed by mutations in β-catenin, the selection of this cell line provided us the tool to gain an insight into targeting of β-catenin signaling by silibinin in its anticancer efficacy against CRC.
APC and β-catenin proteins are important players in Wnt/β-catenin pathway, which is often deregulated in CRC [35]. β-Catenin is a key protein in the canonical Wnt pathway where it acts as a transcriptional coactivator and affects the expression of genes involved in cell proliferation, differentiation, and survival [26]. Because it is involved in the maintenance of cellular homeostasis, both intracellular pool and subcellular localization of β-catenin are critically maintained. In the absence of the Wnt signal, the cytoplasmic pool of β-catenin is maintained at a low level and is regulated by three different pathways. The first canonical pathway involves multiprotein destruction complex, consisting of GSK-3β, casein kinase 1, APC, and axin, which targets β-catenin for proteasomal degradation [36]; the second pathway involves p53/Siah1 [37]; and the third pathway involves nuclear hormone receptor-mediated degradation of β-catenin [38]. APC gene mutations are observed in 70% cases of CRC and are considered as an initiating event as a consequence of the inability of the destruction complex to promote degradation of β-catenin [39,40]. In our study, silibinin significantly inhibited the growth of SW480 cells in culture without causing any considerable death at lower concentrations (50 and 100 εM). At a molecular level, silibinin decreased the total cellular pool as well as the subcellular localization of β-catenin. A decrease in both cytoplasmic and nuclear localization of β-catenin was observed on silibinin treatment, which may reflect an overall decrease in the cellular pool of β-catenin. At an early treatment time, silibinin seems to decrease only the nuclear level of β-catenin; however, at later treatment time points, the effect was evident in both nuclear and cytoplasmic compartments. The prominent decrease in nuclear localization of β-catenin was further corroborated by a decrease in the β-catenin-mediated transcriptional activity after silibinin treatment. Thus, the down-regulation of β-catenin/TCF signaling by silibinin seems to exert an important role in inhibiting the proliferative capacity of CRC cells. The specificity of the growth-inhibitory effect of silibinin through a decrease in β-catenin level and associated downstream signaling was further supported by the findings where HCT116 cells, which harbor wild type APC but mutant β-catenin, failed to respond to both growth inhibition and decrease in β-catenin levels by silibinin.
Recently, Firestein et al. [32] showed that β-catenin transcriptional activity is positively regulated by the kinase activity of CDK8 and identified it as a CRC oncogene. They also reported that SW480 cell line carry gain in copy number for CDK8 gene together with a higher expression of CDK8 protein. CDK8, along with cyclin C, Med12, and Med13, forms a “mediator complex” that is involved in the regulation of transcription [41]. Furthermore, Morris et al. [42] showed that CDK8 could also indirectly upregulate β-catenin-dependent signaling by repressing E2F1 activity; E2F1 directly inhibits β-catenin-dependent transcriptional activity independent of APC/GSK-3β activity. In our study, silibinin treatment decreased the protein level of CDK8, which might have, in part, contributed to the observed inhibition of β-catenin-mediated transcriptional activity by silibinin. Further studies in future, however, are required to fully illustrate this mechanism.
One of the downstream targets of β-catenin/TCF/LEF transcriptional activity involved in cell cycle regulation is cyclin D1, a key molecule facilitating the progression of cells through the G1 checkpoint [43,44]. Another downstream target of this pathway is C-Myc, which is a proto-oncogene that transcriptionally regulates the genes involved in cell cycle progression (G1-S transition), metabolism, ribosome biogenesis, protein synthesis, and apoptosis [45]. We observed a decreased expression of both cyclin D1 and c-Myc by silibinin, suggesting their possible role in the observed growth-inhibitory effects of silibinin in SW480 cells. Recently, it was reported that c-Myc is involved in APC gene loss-induced phenotypes in intestine [46]. Loss of APC gene imparts crypt progenitor cell-like phenotype to the intestinal enterocytes, which fail to differentiate and migrate to the crypt-villus axis, resulting in enlarged intestinal crypts. However, a combined deletion of APC and c-Myc genes in adult mouse small intestine results in proliferation, differentiation, and migration of intestinal enterocytes to crypt-villus axis [47]. On the basis of these literature reports, it could be suggested that the down-regulation of c-Myc protein level by silibinin might contribute to its efficacy against CRC.
To further substantiate in vitro the growth-inhibitory effects of silibinin on human CRC SW480 cells, we extended our studies to in vivo conditions by implanting SW480 tumor xenografts in athymic nude mice. Silibinin feeding significantly inhibited the growth of SW480 tumor xenografts, which was accompanied by a decrease in the levels of β-catenin and its downstream targets, cyclin D1 and c-Myc. A down-regulation in the expression of CDK8 by silibinin treatment was also observed in these studies, further supporting the notion that CDK8 might be one of the molecular targets of silibinin efficacy. Reduced PCNA positivity of tumors fromsilibinin-fed mice confirmed its in vivo growth-inhibitory effects. We also observed apoptotic cells in tumors from silibinin-fed mice; therefore, induction of apoptosis by silibinin may also contribute to its in vivo efficacy.
In recent years, there had been an effort to translate the relevance of both cell culture and preclinical anticancer efficacy studies into the clinical settings, specifically those related to a comparable effective dose in humans. To address the issue of dose translation from one species to another, calculation of human equivalent dose (HED) using normalization of the body surface area instead of weight has been advocated [48]. On the basis of the formula proposed for calculating HED, we found that the corresponding HED for silibinin at the maximum dose (200 mg/kg body weight of mouse) used in our in vivo study would be 973 mg/60-kg adult. The resultant HED is within the doses used in CRC patients in the study by Hoh et al. [49] where the patients were fed with 360 to 1440 mg/day of silipide (a formulation of silibinin for better bioavailability) for 7 days, and the resultant achievable levels of silibinin in normal and malignant colorectal tissue (target organ) were 78 to 141 nmol/g, which is again within the range of 100-εM concentration of silibinin used in our in vitro experiments. Thus, the effective in vitro concentrations and in vivo doses of silibinin used in the present study are within the range of physiologically achievable concentrations of silibinin in the colon tissue of CRC patients fed with silibinin doses in line with our animal studies. Collectively, the findings of the present study along with no apparent toxicity associated with silibinin underscore the efficacy of silibinin against CRC with translational potential in the future.
Abbreviations
- APC
adenomatous polyposis coli
- CDK8
cyclin-dependent kinase 8
- CRC
colorectal cancer
Footnotes
This work was supported by National Cancer Institute grant RO1 CA112304.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Mandel JS, Church TR, Bond JH, Ederer F, Geisser MS, Mongin SJ, Snover DC, Schuman LM. The effect of fecal occult-blood screening on the incidence of colorectal cancer. N Engl J Med. 2000;343:1603–1607. doi: 10.1056/NEJM200011303432203. [DOI] [PubMed] [Google Scholar]
- 3.Espey DK, Wu XC, Swan J, Wiggins C, Jim MA, Ward E, Wingo PA, Howe HL, Ries LA, Miller BA, et al. Annual report to the nation on the status of cancer, 1975–2004, featuring cancer in American Indians and Alaska Natives. Cancer. 2007;110:2119–2152. doi: 10.1002/cncr.23044. [DOI] [PubMed] [Google Scholar]
- 4.Wilkins T, Reynolds PL. Colorectal cancer: a summary of the evidence for screening and prevention. Am Fam Physician. 2008;78:1385–1392. [PubMed] [Google Scholar]
- 5.Half E, Arber N. Colon cancer: preventive agents and the present status of chemoprevention. Expert Opin Pharmacother. 2009;10:211–219. doi: 10.1517/14656560802560153. [DOI] [PubMed] [Google Scholar]
- 6.Corrêa Lima MP, Gomes-da-Silva MH. Colorectal cancer: lifestyle and dietary factors. Nutr Hosp. 2005;20:235–241. [PubMed] [Google Scholar]
- 7.Coyle YM. Lifestyle, genes, and cancer. Methods Mol Biol. 2009;472:25–56. doi: 10.1007/978-1-60327-492-0_2. [DOI] [PubMed] [Google Scholar]
- 8.Nomura AM, Wilkens LR, Murphy SP, Hankin JH, Henderson BE, Pike MC, Kolonel LN. Association of vegetable, fruit, and grain intakes with colorectal cancer: the Multiethnic Cohort Study. Am J Clin Nutr. 2008;88:730–737. doi: 10.1093/ajcn/88.3.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Duijnhoven FJ, Bueno-De-Mesquita HB, Ferrari P, Jenab M, Boshuizen HC, Ros MM, Casagrande C, Tjønneland A, Olsen A, Overvad K, et al. Fruit, vegetables, and colorectal cancer risk: the European Prospective Investigation into Cancer and Nutrition. Am J Clin Nutr. 2009;89:1441–1452. doi: 10.3945/ajcn.2008.27120. [DOI] [PubMed] [Google Scholar]
- 10.Millen AF, Subar AF, Graubard BI, Peters U, Hayes RB, Weissfeld JL, Yokochi LA, Ziegler RG, PLCO Cancer Screening Trial Project Team Fruit and vegetable intake and prevalence of colorectal adenoma in a cancer screening trial. Am J Clin Nutr. 2007;86:1754–1764. doi: 10.1093/ajcn/86.5.1754. [DOI] [PubMed] [Google Scholar]
- 11.Mann CD, Neal CP, Garcea G, Manson MM, Dennison AR, Berry DP. Phytochemicals as potential chemopreventive and chemotherapeutic agents in hepatocarcinogenesis. Eur J Cancer Prev. 2009;18:13–25. doi: 10.1097/CEJ.0b013e3282f0c090. [DOI] [PubMed] [Google Scholar]
- 12.Khor TO, Yu S, Kong AN. Dietary cancer chemopreventive agents—targeting inflammation and Nrf2 signaling pathway. Planta Med. 2008;74:1540–1547. doi: 10.1055/s-0028-1088303. [DOI] [PubMed] [Google Scholar]
- 13.Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3:768–780. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 14.Kaur M, Singh RP, Gu M, Agarwal R, Agarwal C. Grape seed extract inhibits in vitro and in vivo growth of human colorectal carcinoma cells. Clin Cancer Res. 2006;12:6194–6202. doi: 10.1158/1078-0432.CCR-06-1465. [DOI] [PubMed] [Google Scholar]
- 15.Velmurugan B, Singh RP, Tyagi A, Agarwal R. Inhibition of azoxymethane-induced colonic aberrant crypt foci formation by silibinin in male Fisher 344 rats. Cancer Prev Res (Phila Pa) 2008;1:376–384. doi: 10.1158/1940-6207.CAPR-08-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh RP, Gu M, Agarwal R. Silibinin inhibits colorectal cancer growth by inhibiting tumor cell proliferation and angiogenesis. Cancer Res. 2008;68:2043–2050. doi: 10.1158/0008-5472.CAN-07-6247. [DOI] [PubMed] [Google Scholar]
- 17.Singh RP, Agarwal R. Prostate cancer chemoprevention by silibinin: bench to bedside. Mol Carcinog. 2006;45:436–442. doi: 10.1002/mc.20223. [DOI] [PubMed] [Google Scholar]
- 18.Singh RP, Agarwal R. Mechanisms and preclinical efficacy of silibinin in preventing skin cancer. Eur J Cancer. 2005;41:1969–1979. doi: 10.1016/j.ejca.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 19.Agarwal C, Singh RP, Dhanalakshmi S, Tyagi AK, Tecklenburg M, Sclafani RA, Agarwal R. Silibinin upregulates the expression of cyclin-dependent kinase inhibitors and causes cell cycle arrest and apoptosis in human colon carcinoma HT-29 cells. Oncogene. 2003;22:8271–8282. doi: 10.1038/sj.onc.1207158. [DOI] [PubMed] [Google Scholar]
- 20.Tyagi A, Raina K, Singh RP, Gu M, Agarwal C, Harrison G, Glode LM, Agarwal R. Chemopreventive effects of silymarin and silibinin on N-butyl-N-(4-hydroxybutyl) nitrosamine induced urinary bladder carcinogenesis in male ICR mice. Mol Cancer Ther. 2007;6:3248–3255. doi: 10.1158/1535-7163.MCT-07-2006. [DOI] [PubMed] [Google Scholar]
- 21.Raina K, Blouin MJ, Singh RP, Majeed N, Deep G, Varghese L, Glodé LM, Greenberg NM, Hwang D, Cohen P, et al. Dietary feeding of silibinin inhibits prostate tumor growth and progression in transgenic adenocarcinoma of the mouse prostate model. Cancer Res. 2007;67:11083–11091. doi: 10.1158/0008-5472.CAN-07-2222. [DOI] [PubMed] [Google Scholar]
- 22.Singh RP, Deep G, Chittezhath M, Kaur M, Dwyer-Nield LD, Malkinson AM, Agarwal R. Effect of silibinin on the growth and progression of primary lung tumors in mice. J Natl Cancer Inst. 2006;98:846–855. doi: 10.1093/jnci/djj231. [DOI] [PubMed] [Google Scholar]
- 23.Moon RT, Kohn AD, De Ferrari GV, Kaykas A. WNT and β-catenin signalling: diseases and therapies. Nat Rev Genet. 2004;5:691–701. doi: 10.1038/nrg1427. [DOI] [PubMed] [Google Scholar]
- 24.Gottardi CJ, Gumbiner BM. Adhesion signaling: how β-catenin interacts with its partners. Curr Biol. 2001;11:R792–R794. doi: 10.1016/s0960-9822(01)00473-0. [DOI] [PubMed] [Google Scholar]
- 25.Barker NV. The canonical Wnt/β-catenin signalling pathway. Methods Mol Biol. 2008;468:5–15. doi: 10.1007/978-1-59745-249-6_1. [DOI] [PubMed] [Google Scholar]
- 26.Takemaru KI, Ohmitsu M, Li FQ. An oncogenic hub: β-catenin as a molecular target for cancer therapeutics. Handb Exp Pharmacol. 2008;186:261–284. doi: 10.1007/978-3-540-72843-6_11. [DOI] [PubMed] [Google Scholar]
- 27.Fodde R. The APC gene in colorectal cancer. Eur J Cancer. 2002;38:867–871. doi: 10.1016/s0959-8049(02)00040-0. [DOI] [PubMed] [Google Scholar]
- 28.Ilyas M, Tomlinson IP, Rowan A, Pignatelli M, Bodmer WF. β-Catenin mutations in cell lines established from human colorectal cancers. Proc Natl Acad Sci USA. 1997;94:10330–10334. doi: 10.1073/pnas.94.19.10330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler KW. Activation of β-catenin-Tcf signaling in colon cancer by mutations in β-catenin or APC. Science. 1997;275:1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- 30.Rowan AJ, Lamlum H, Ilyas M, Wheeler J, Straub J, Papadopoulou A, Bicknell D, Bodmer WF, Tomlinson IP. APC mutations in sporadic colorectal tumors: a mutational “hotspot” and interdependence of the “two hits”. Proc Natl Acad Sci USA. 2000;97:3352–3357. doi: 10.1073/pnas.97.7.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dhanalakshmi S, Singh RP, Agarwal C, Agarwal R. Silibinin inhibits constitutive and TNFα-induced activation of NF-κB and sensitizes human prostate carcinoma DU145 cells to TNFα-induced apoptosis. Oncogene. 2002;21:1759–1767. doi: 10.1038/sj.onc.1205240. [DOI] [PubMed] [Google Scholar]
- 32.Firestein R, Bass AJ, Kim SY, Dunn IF, Silver SJ, Guney I, Freed E, Ligon AH, Vena N, Ogino S, et al. CDK8 is a colorectal cancer oncogene that regulates β-catenin activity. Nature. 2008;455:547–551. doi: 10.1038/nature07179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davis-Searles PR, Nakanishi Y, Kim NC, Graf TN, Oberlies NH, Wani MC, Wall ME, Agarwal R, Kroll DJ. Milk thistle and prostate cancer: differential effects of pure flavonolignans from Silybum marianum on antiproliferative end points in human prostate carcinoma cells. Cancer Res. 2005;65:4448–4457. doi: 10.1158/0008-5472.CAN-04-4662. [DOI] [PubMed] [Google Scholar]
- 34.Luper S. A review of plants used in the treatment of liver disease: part 1. Altern Med Rev. 1998;3:410–421. [PubMed] [Google Scholar]
- 35.Schneikert J, Behrens J. The canonical Wnt signalling pathway and its APC partner in colon cancer development. Gut. 2007;56:417–425. doi: 10.1136/gut.2006.093310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kimelman D, Xu W. β-Catenin destruction complex: insights and questions from a structural perspective. Oncogene. 2006;25:7482–7491. doi: 10.1038/sj.onc.1210055. [DOI] [PubMed] [Google Scholar]
- 37.Liu J, Stevens J, Rote CA, Yost HJ, Hu Y, Neufeld KL, White RL, Matsunami N. Siah-1 mediates a novel β-catenin degradation pathway linking p53 to the adenomatous polyposis coli protein. Mol Cell. 2001;7:927–936. doi: 10.1016/s1097-2765(01)00241-6. [DOI] [PubMed] [Google Scholar]
- 38.Dillard AC, Lane MA. Retinol decreases β-catenin protein levels in retinoic acid-resistant colon cancer cell lines. Mol Carcinog. 2007;46:315–329. doi: 10.1002/mc.20280. [DOI] [PubMed] [Google Scholar]
- 39.Nagase H, Nakamura Y. Mutations of the APC (adenomatous polyposis coli) gene. Hum Mutat. 1993;2:425–434. doi: 10.1002/humu.1380020602. [DOI] [PubMed] [Google Scholar]
- 40.Moon RT, Miller JR. The APC tumor suppressor protein in development and cancer. Trends Genet. 1997;13:256–258. doi: 10.1016/s0168-9525(97)01196-7. [DOI] [PubMed] [Google Scholar]
- 41.Conaway RC, Sato S, Tomomori-Sato C, Yao T, Conaway JW. The mammalian Mediator complex and its role in transcriptional regulation. Trends Biochem Sci. 2005;30:250–255. doi: 10.1016/j.tibs.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 42.Morris EJ, Ji JY, Yang F, Di Stefano L, Herr A, Moon NS, Kwon EJ, Haigis KM, Näär AM, Dyson NJ. E2F1 represses β-catenin transcription and is antagonized by both pRB and CDK8. Nature. 2008;455:552–556. doi: 10.1038/nature07310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Behrens J. Control of β-catenin signaling in tumor development. Ann N Y Acad Sci. 2000;910:21–33. doi: 10.1111/j.1749-6632.2000.tb06698.x. [DOI] [PubMed] [Google Scholar]
- 44.Fu M, Wang C, Li Z, Sakamaki T, Pestell RG. Minireview: cyclin D1: normal and abnormal functions. Endocrinology. 2004;145:5439–5447. doi: 10.1210/en.2004-0959. [DOI] [PubMed] [Google Scholar]
- 45.Nasi S, Ciarapica R, Jucker R, Rosati J, Soucek L. Making decisions through Myc. FEBS Lett. 2001;490:153–162. doi: 10.1016/s0014-5793(01)02118-4. [DOI] [PubMed] [Google Scholar]
- 46.Wilkins JA, Sansom OJ. C-Myc is a critical mediator of the phenotypes of APC loss in the intestine. Cancer Res. 2008;68:4963–4966. doi: 10.1158/0008-5472.CAN-07-5558. [DOI] [PubMed] [Google Scholar]
- 47.Sansom OJ, Meniel VS, Muncan V, Phesse TJ, Wilkins JA, Reed KR, Vass JK, Athineos D, Clevers H, Clarke AR. Myc deletion rescues APC deficiency in the small intestine. Nature. 2007;446:676–679. doi: 10.1038/nature05674. [DOI] [PubMed] [Google Scholar]
- 48.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 49.Hoh C, Boocock D, Marczylo T, Singh R, Berry DP, Dennison AR, Hemingway D, Miller A, West K, Euden S, et al. Pilot study of oral silibinin, a putative chemopreventive agent, in colorectal cancer patients: silibinin levels in plasma, colorectum, and liver and their pharmacodynamic consequences. Clin Cancer Res. 2006;12:2944–2950. doi: 10.1158/1078-0432.CCR-05-2724. [DOI] [PubMed] [Google Scholar]